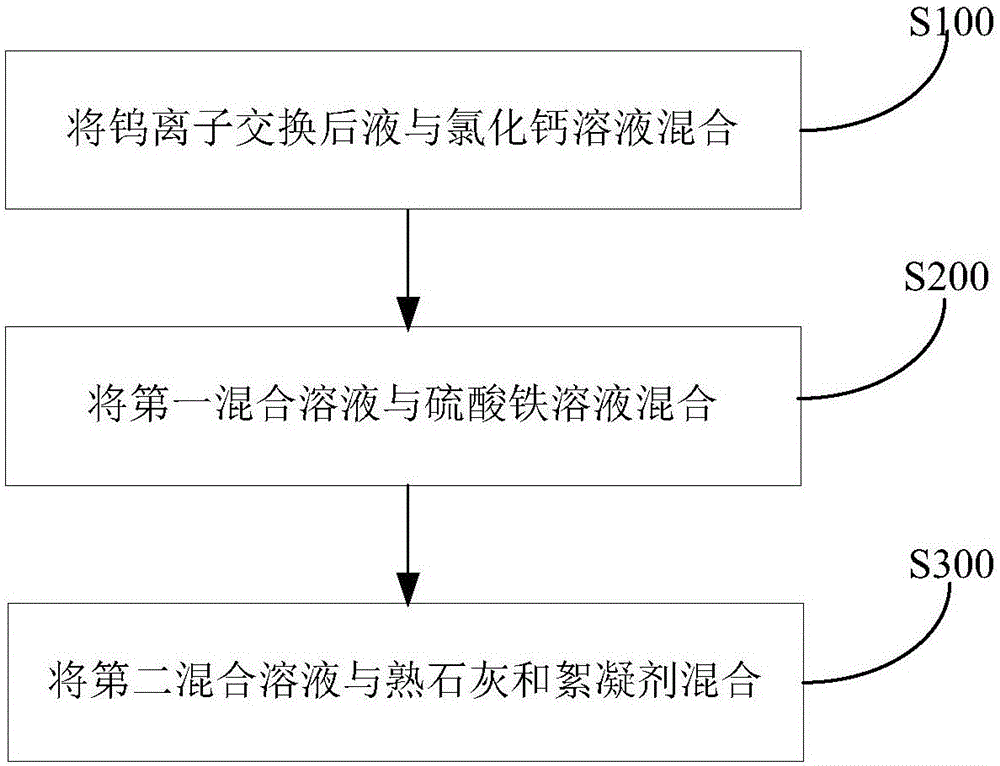
Method for removing fluorine in liquid after tungsten ion exchange
An ion exchange and fluoride ion technology, which is applied in the field of removing fluorine in the liquid after tungsten ion exchange, can solve the problems such as the inability to achieve the fluorine removal effect, and achieve the effect of high stability and simple process flow.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
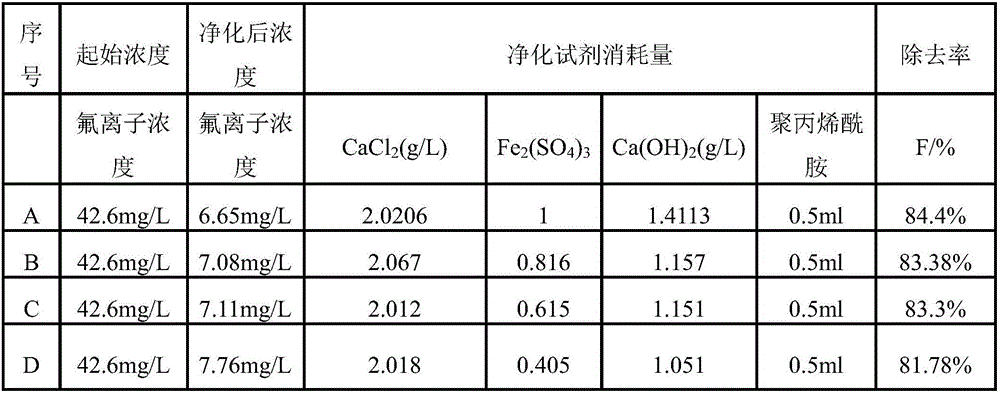
[0052] Take 500ml of tungsten ion-exchanged liquid with a fluorine concentration of 42.6mg / L, divide it into four groups A, B, C, and D, and then add CaCl according to the reagent dosage shown in Table 1 2 And mix and stir for 10min, the stirring speed is 100r / min, then add Fe 2 (SO 4 ) 3 , stirred at room temperature for 10min at a stirring speed of 100r / min, and finally added Ca(OH) 2 Precipitate and adjust the pH, the stirring speed is 100r / min, the stirring time is 5min, and then add 0.5ml of polyacrylamide flocculant.
[0053] Table 1 Reagent dosage and fluorine concentration
[0054]
Embodiment 2
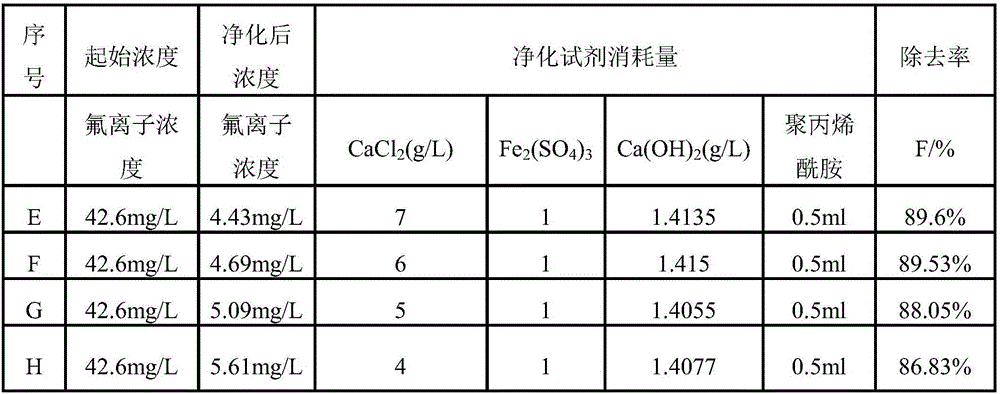
[0056] Take 500ml of tungsten ion-exchanged solution with a fluorine concentration of 42.6mg / L, divide it into four groups E, F, G, and H, and then add CaCl respectively according to the reagent dosage shown in Table 2 2 And mix and stir for 10min, the stirring speed is 300r / min, then add Fe 2 (SO 4 ) 3 , stirred at room temperature for 10min at a stirring speed of 300r / min, and finally added Ca(OH) 2 Precipitate and adjust the pH, the stirring speed is 300r / min, the stirring time is 10min, and then add 0.5ml of polyacrylamide flocculant.
[0057] Table 2 Reagent dosage and fluorine concentration
[0058]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com