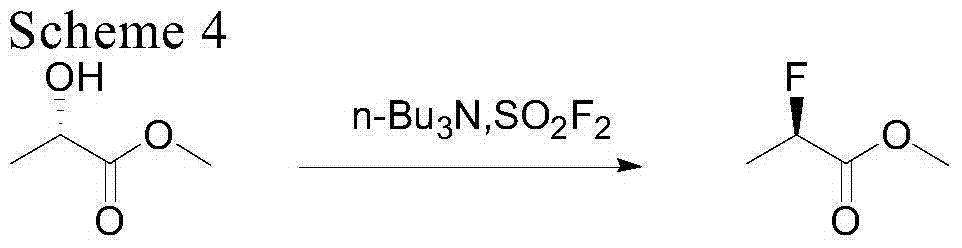Synthesis method of R-2-fluorine methyl lactate
A kind of technology of methyl fluorolactate and synthetic method, applied in the field of synthetic route of industrial production, can solve problems such as not mentioned
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
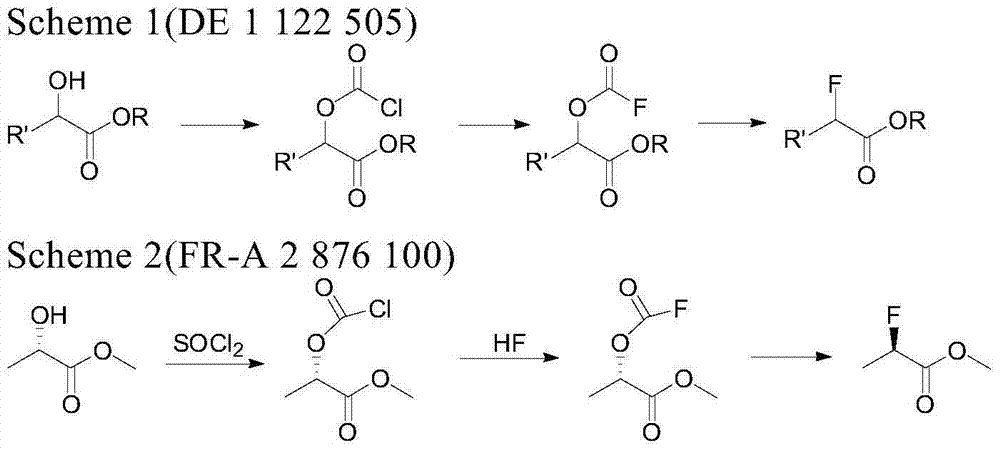
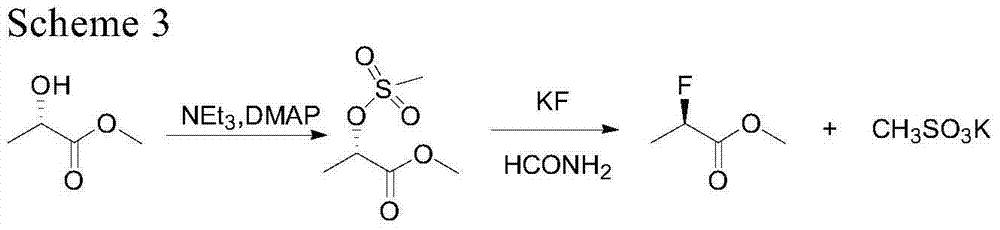
[0030] The invention relates to a method for synthesizing R-2-fluoromethyl lactate. This method is to obtain R-2-fluorolactate methyl ester by S-methyl lactate in the presence of an organic base, by selectively feeding or not feeding hydrogen fluoride gas, fluorinating with sulfuryl fluoride, and then short-distilling and rectifying. Organic bases can be recovered and reused without reducing reactivity. The method has high yield and high ee value, only produces few "three wastes", and the raw materials are cheap and easy to obtain.
[0031] Compound R-2-fluoromethyl lactate of the present invention takes S-methyl lactate as a raw material, and it is speculated that it is obtained by the following mechanism:
[0032]
[0033] A preferred embodiment of the present invention is,
[0034] The distillation residue is added with an inorganic base to recover N,N-dimethylaniline, which can be reused without reducing the reactivity.
[0035] Specifically, the synthetic method of ...
Embodiment 1
[0062] The following examples will help to further understand the present invention, but do not limit the content of the present invention.
[0063] Step 1: Reaction and purification of R-2-fluoromethyl lactate
[0064] Add 1249.2g of S-methyl lactate and 2669g of tri-n-butylamine into a 5L reaction flask, cool down to -10°C, keep warm at -10-0°C and feed 1286g of sulfonyl fluoride gas until the reaction of S-methyl lactate is complete. The crude product was distilled off and rectified to obtain 1056.5 g of methyl R-2-fluorolactate with a yield of 83%. The content determined by gas chromatography was 99.8%, and the ee value was 97.24%. The residue of the kettle is 3945g.
[0065] The recovery of step two n-tributylamine one
[0066] 3945g of the still residue obtained in step 1 was heated to 60°C, and 4160g of 15% sodium hydroxide solution was added dropwise. After the dropwise addition was completed, the liquid was separated after heat preservation to obtain 5445 g of the ...
Embodiment 2-7
[0072] The consumption of organic base and S-methyl lactate is changed, others are the same as Example 1, and the results are shown in Table 1
[0073] Table 1:
[0074] Example
PUM
| Property | Measurement | Unit |
|---|---|---|
| optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com