Preparation method of 1-difluoroalkyl isoquinoline
A technology of difluoroalkylisoquinoline and difluoroacetic acid, which is applied in the field of preparation of 1-difluoroalkylisoquinoline, and achieves the effects of mild reaction conditions, strong operability, high reaction conversion rate and yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
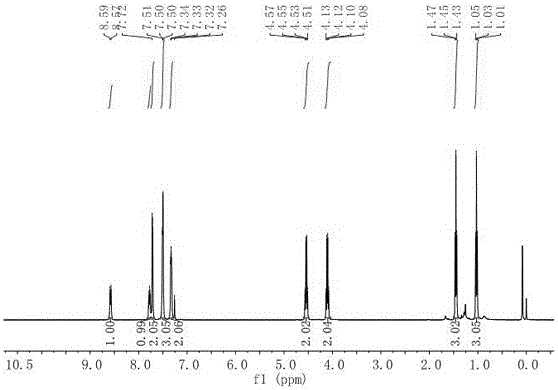
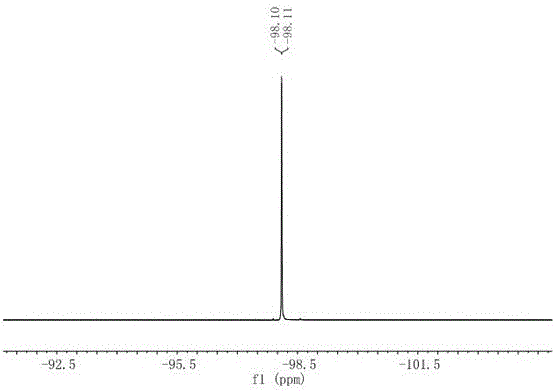
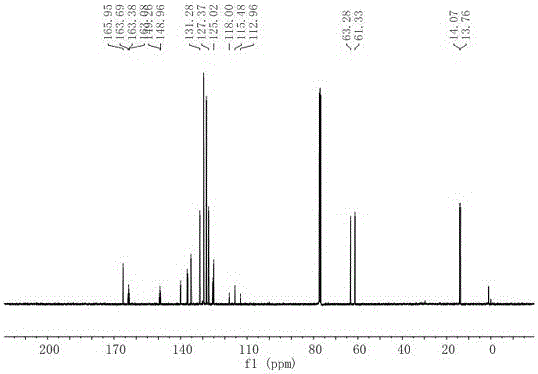
[0024] Preparation of compound 1-(2-ethoxy-1,1-difluoro-2-oxoethyl)-4-phenylisoquinoline-3-carboxylic acid ethyl ester:
[0025]
[0026] Add palladium chloride (53mg, 0.3mmol, 10mol%), 1,2-bis(diphenylphosphino)ethane (239mg, 0.6mmol, 20mol%) to a reaction tube protected by dry oxygen-free nitrogen. Potassium carbonate (828mg, 6mmol, 2.0equiv.), followed by methyl 2-isocyano-3,3-diphenylacrylate (2.5g, 9mmol, 3.0equiv.), ethyl bromodifluoroacetate (610mg, 3mmol , 1.0 equiv.) and anhydrous N-methylpyrrolidone (10 mL) were added. The reaction flask was placed in an oil bath at 80°C for full stirring for 12 hours. After confirming the end of the reaction by thin layer chromatography, cool the reaction flask to room temperature, add an appropriate amount of aqueous solution to quench the reaction, add ethyl acetate to extract the aqueous phase, combine the organic phases, wash the organic phases with saturated aqueous NaCl, and dry with anhydrous sodium sulfate The solvent was rec...
Embodiment 2
[0029] Preparation of compound 1-(2-ethoxy-1,1-difluoro-2-oxoethyl)-4-methylisoquinoline-3-carboxylic acid ethyl ester:
[0030]
[0031] Add palladium chloride (53mg, 0.3mmol, 10mol%), 1,2-bis(diphenylphosphino)ethane (239mg, 0.6mmol, 20mol%) to a reaction tube protected by dry oxygen-free nitrogen. Potassium carbonate (828mg, 6mmol, 2.0equiv.), followed by ethyl (Z)-2-isocyano-3-p-tolylbut-2-enoate (1.95g, 9mmol, 3.0equiv.), bromodifluoro Ethyl acetate (610 mg, 3 mmol, 1.0 equiv.) and anhydrous N-methylpyrrolidone (10 mL) were added. The reaction flask was placed in an oil bath at 80°C for full stirring for 12 hours. After confirming the end of the reaction by thin layer chromatography, cool the reaction flask to room temperature, add an appropriate amount of aqueous solution to quench the reaction, add ethyl acetate to extract the aqueous phase, combine the organic phases, wash the organic phases with saturated aqueous NaCl, and dry with anhydrous sodium sulfate The solvent ...
Embodiment 3
[0034] Preparation of compound ethyl 1-(2-(diethylamino)-1,1-difluoro-2-oxoethyl)-4-phenylisoquinoline-3-carboxylic acid ethyl ester:
[0035]
[0036] Add palladium chloride (53mg, 0.3mmol, 10mol%), 1,2-bis(diphenylphosphino)ethane (239mg, 0.6mmol, 20mol%) to a reaction tube protected by dry oxygen-free nitrogen. Potassium carbonate (828mg, 6mmol, 2.0equiv.), followed by methyl 2-isocyano-3,3-diphenylacrylate (2.5g, 9mmol, 3.0equiv.), 2-bromo-N,N-diethyl Benzyl-2,2-difluoroacetamide (687 mg, 3 mmol, 1.0 equiv.) and anhydrous N-methylpyrrolidone (10 mL) were added. The reaction flask was placed in an oil bath at 80°C for full stirring for 12 hours. After confirming the end of the reaction by thin layer chromatography, cool the reaction flask to room temperature, add an appropriate amount of aqueous solution to quench the reaction, add ethyl acetate to extract the aqueous phase, combine the organic phases, wash the organic phases with saturated aqueous NaCl, and dry with anhydrou...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com