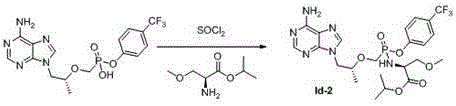
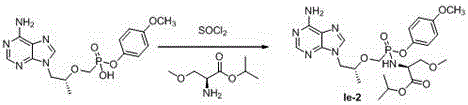
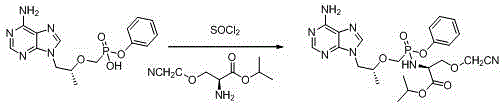
Phosphonate prodrug of adenine derivative and medical application of phosphonate prodrug
A technology for medicinal salts and drugs, applied in the field of phosphonate prodrugs or isomers thereof, can solve the problems of renal toxicity, potential safety hazards and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Embodiment 1: the preparation of O-methyl-serine isopropyl ester
[0026]
[0027] step 1:
[0028] Add 5.00g of serine to 50mL of isopropanol, add 11.32g of SOCl dropwise 2 . After the reaction was refluxed overnight, it was directly concentrated to obtain 8.47 g of white serine isopropyl ester hydrochloride with a yield of 96.9%.
[0029] Step 2:
[0030] Get in the 6.00g serine isopropyl ester hydrochloride DCM that obtains in the step 1 of above-mentioned embodiment 1, then dropwise add 8.25gEt 3 N. Add 8.64g Boc under ice bath 2 O, and react overnight at room temperature. Add water, separate the layers, wash the organic layer with brine, dry, and pass through the column to obtain 7.41 g of white solid (N-tert-butoxycarbonyl-serine isopropyl ester), with a yield of 91.7%.
[0031] Step 3:
[0032] Take 7.36g of N-tert-butoxycarbonyl-serine isopropyl ester obtained in step 2 of the above example 1 and add it to 147mL of DCM, then add 35.46g of silver oxide...
Embodiment 2
[0035] Example 2: Preparation of 9-[(R)-2-(phenylphosphonomethoxy)propyl]adenine
[0036]
[0037] Add 1.01g tenofovir (PMPA) into 14mL pyridine at room temperature, add 3.24g triphenyl phosphite into the reaction solution at room temperature, heat to reflux temperature 115°C for 8 hours, the solution becomes clear first, then A large amount of white solid precipitated, cooled to about -5°C to 5°C, added 14mL of acetone, stirred for 1.5 hours, filtered, washed the filter cake with 1mL of acetone and dried at 60°C to 70°C for 5 hours, the obtained white solid was the obtained target product , giving a total of 1.12 g of 9-[(R)-2-(phenylphosphonomethoxy)propyl]adenine as a white solid.
Embodiment 3
[0038] Embodiment 3: the synthesis of compound Ia-1
[0039]
[0040] Get the 0.67g 9-[(R)-2-(phenylphosphonomethoxy) propyl group] adenine obtained in Example 2 and add 5mLSOCl 2 In, reflux reaction for 2 hours, concentrated for later use. Take 1.30 g of O-methyl-serine isopropyl ester and 0.36 g of triethylamine obtained in Step 4 of Example 1 and add them into 10 mL of DCM, cool down to -10° C., and then add the above-mentioned concentrate to be used. After the addition, react at -10°C for about 2h, then move to room temperature and stir for about 1h. After concentration, 0.75 g of compound Ia-1 was obtained with a yield of 80.6%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com