Synthesis method of 3-oxetane acetic acid
A technology of oxetane and synthesis method, which is applied in the direction of organic chemistry, can solve the problems of no literature report on compound synthesis methods, etc., and achieve the effects of avoiding product deterioration, reasonable route design, and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Embodiment 1: small test experiment
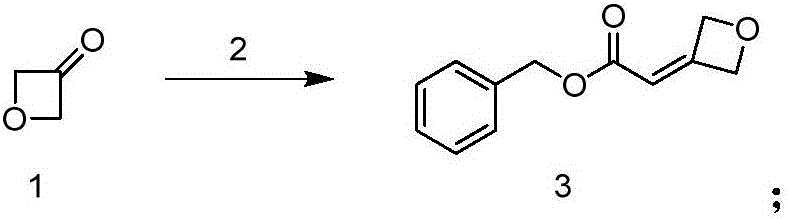
[0023] first step:
[0024]
[0025] Table 1 Step one small test experimental feeding table
[0026] compound molecular weight Feeding amount relative molar mass Equivalent number 1 72 4g 0.056mol 1.1eq 2 410 20.7g 0.051mol 1.0eq THF - 80mL - -
[0027] The dosages of various compounds in this reaction are shown in Table 1.
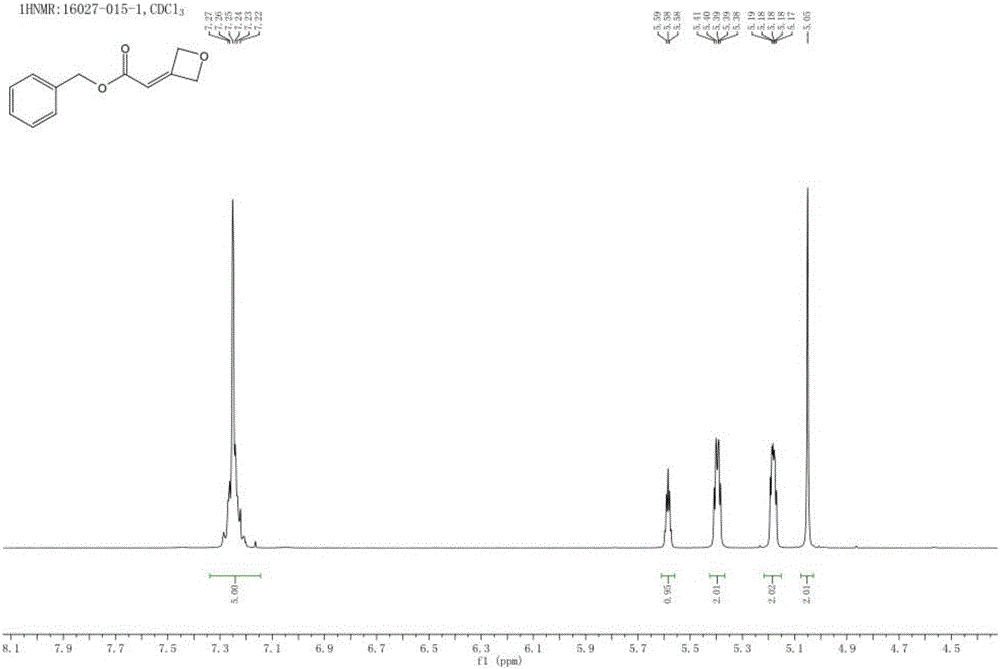
[0028] Operation steps: Add compound 2 (20.7g) and THF (50mL) into a 250mL three-necked flask in sequence, drop the mixture of compound 1 (4g) and THF (30mL) into the system at 0-5°C, and stir at natural temperature After 5 hours, TLC (PE:EA=10:1) showed that the reaction of the starting material (Rf=0.1) was complete, and a product (Rf=0.3) was formed.
[0029] Post-processing: the above system was spin-dried, purified by column chromatography (PE:EA=20:1) to obtain the product point, and spin-dried to obtain compound 3 (8 g of white solid), with a yiel...
Embodiment 2
[0040] Embodiment 2: pilot test
[0041] first step:
[0042]
[0043] Table 3 Step 1 Pilot Test Feeding Table
[0044] compound molecular weight Feeding amount relative molar mass Equivalent number 1 72 40g 0.56mol 1.1eq 2 410 207g 0.51mol 1.0eq THF - 800mL - -
[0045] The dosages of various compounds in this reaction are shown in Table 3.
[0046] Operation steps: Add compound 2 (207g) and THF (500mL) into a 2L three-necked flask in turn, drop the mixture of compound 1 (40g) and THF (300mL) into the system at 0-5°C, and stir at natural temperature for 7 hours , TLC (PE:EA=10:1) showed that the reaction of the starting material (Rf=0.1) was completed, and a product (Rf=0.3) was formed.
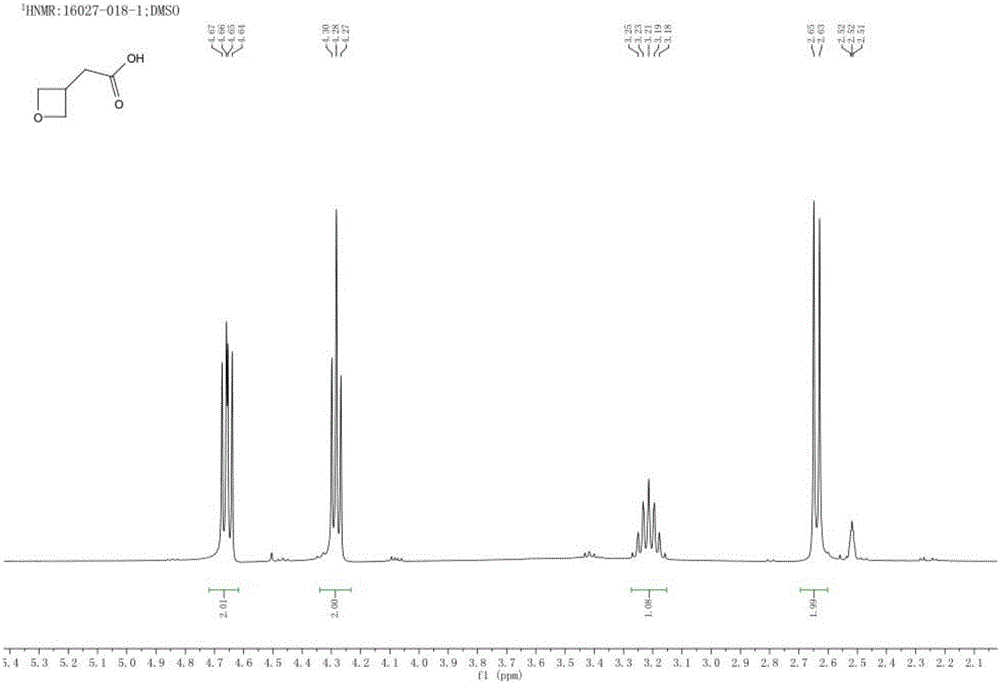
[0047] Post-processing: the above system was spin-dried, purified by column chromatography (PE:EA=20:1) to obtain the product point, and spin-dried to obtain compound 3 (75 g of white solid), with a yield of 61%.
[0048] Step two: ...
Embodiment 3
[0056] Embodiment 3: scale-up experiment
[0057] first step:
[0058]
[0059] Table 5 Step 1 scale-up experiment feed list
[0060] compound molecular weight Feeding amount relative molar mass Equivalent number 1 72 200g 2.7mol 1.2eq 2 410 1.04kg 2.4mol 1.0eq THF - 3mL - -
[0061] The feeding amount of various compounds in this reaction is shown in Table 5.
[0062]Operation steps: Add compound 2 (1.04kg) and THF (2mL) into a 5L three-necked flask in turn, drop the mixture of compound 1 (200g) and THF (1L) into the system at 0-5°C, and stir at natural temperature for 10 After 1 hour, TLC (PE:EA=10:1) showed that the reaction of the starting material (Rf=0.1) was complete, and a product (Rf=0.3) was formed.
[0063] Post-processing: the above system was spin-dried, purified by column chromatography (PE:EA=20:1) to obtain the product point, and spin-dried to obtain compound 3 (420 g of white solid), with a yield of 68...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com