Application of aryl substitute acryl triterpenoids to preparation of anticomplement drugs
A technology of acryloyl triterpenoids and compounds, which is applied in the field of traditional Chinese medicine and pharmaceuticals, and can solve problems that have not yet been seen
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Embodiment 1. Preparation of aryl-substituted acryloyl triterpenoids
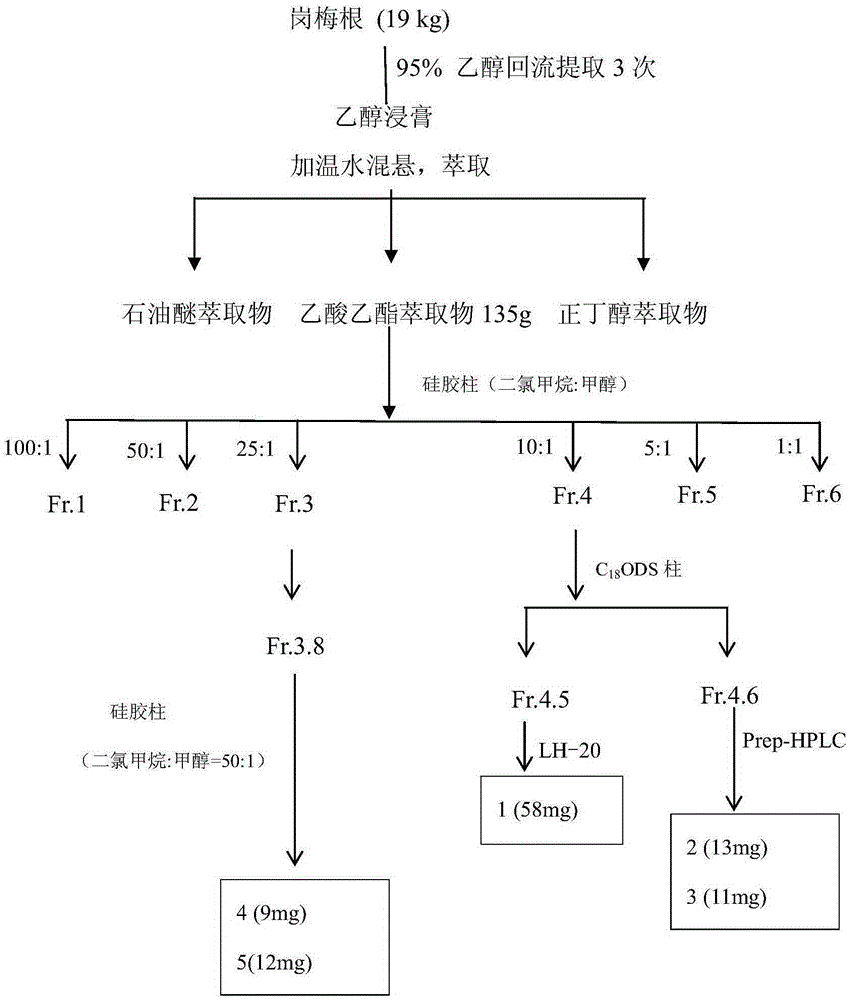
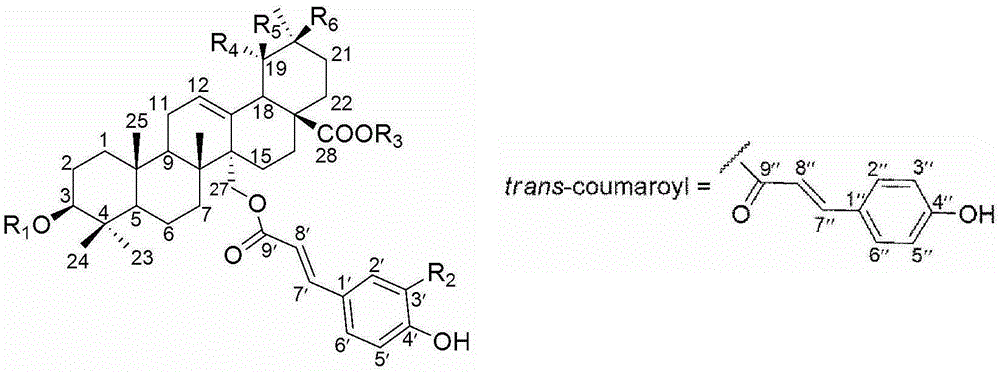
[0032]Take 19kg of Plum root, heat and reflux with 95% ethanol to extract 3 times, suspend the extract with water after concentration, extract with petroleum ether, ethyl acetate, and n-butanol respectively to obtain 135g of ethyl acetate in the active part, take 120g of the extract for extraction Silica gel column chromatography, eluting with dichloromethane-methanol gradient (100:1→1:1). The fraction of Fr.4 eluted with dichloromethane-methanol (10:1) was then passed through an ODS reverse column, followed by gradient elution with methanol / water (20% → 100%), with ultraviolet 210nm as the detection wavelength, and a total of Fr .4 (1-6) 6 fractions, Fr.3 also carried out gradient elution through ODS column methanol / water (30% → 100%) to obtain Fr.3 (1-8) fractions, Fr.4.5 again Using a silica gel column dichloromethane-acetone-methanol (20:2:1) for isocratic elution and separation, the compound As...
Embodiment 2
[0033] Example 2. Anti-complement classical pathway test in vitro
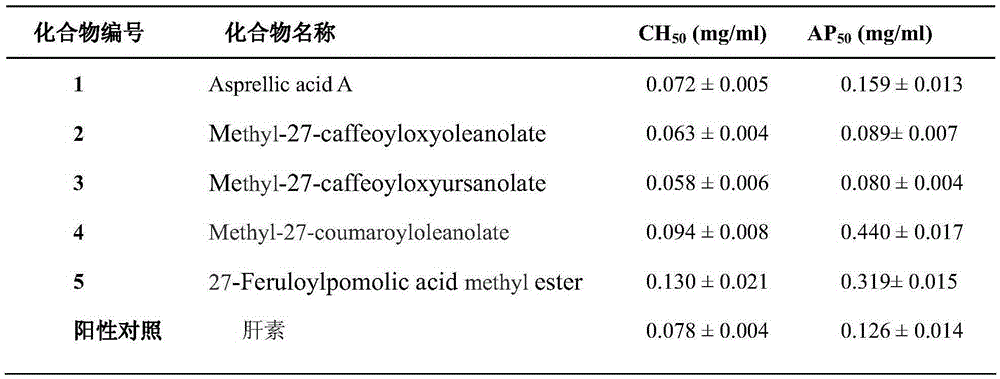
[0034] Take 0.1ml of complement (guinea pig serum), add barbiturate buffer solution (BBS) to prepare a 1:10 solution, and double-dilute with BBS to 1:20, 1:40, 1:80, 1:160, 1:10 320, 1:640 and 1:1280 solutions. Take 1:1000 hemolysin, 0.1ml of each concentration of complement and 2% sheep red blood cells (SRBC) and dissolve them in 0.3ml of BBS, mix well, put them in a low-temperature high-speed centrifuge after 30 minutes in a water bath at 37°C, and put them in a low-temperature high-speed centrifuge at 5000rpm and 4°C. Centrifuge for 10 min. Take 0.2ml of the supernatant from each tube and place it in a 96-well plate, and measure its absorbance at 405nm. At the same time, a complete hemolysis group (0.1ml 2% SRBC dissolved in 0.5ml triple distilled water) was set up in the experiment. The absorbance of three-distilled water lysed blood vessels was used as the standard of total hemolysis, and the hemolysis...
Embodiment 3
[0035] Example 3. Anti-complement alternative pathway test in vitro
[0036] Take 0.2ml of complement (human serum), add AP diluent (barbital buffer, pH=7.4, containing 5mMMg 2+ ,8mM EGTA) was prepared as a 1:1 solution, and double-diluted into 1:2, 1:4, 1:8, 1:16, 1:32, 1:64 and 1:128 solutions. Take 0.15ml of complement of each concentration, 0.15ml of AP diluent and 0.20ml of 0.5% rabbit erythrocytes (RE), mix them evenly, put them in a low-temperature high-speed centrifuge at 37°C for 30min, and centrifuge at 5000rpm and 4°C for 10min. Take 0.2ml of the supernatant from each tube and place it in a 96-well plate, and measure the absorbance at 405nm. At the same time, a complete hemolysis group (0.20ml 0.5% RE dissolved in 0.3ml triple distilled water) was set up in the experiment. The absorbance of three-distilled water lysed blood vessels was used as the standard of total hemolysis, and the hemolysis rate was calculated. Plot the dilution of complement on the X-axis and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com