Preparation and testing method of a three-dimensional decompression device for avascular necrosis of the femoral head
A technology of decompression device and testing method, which is applied in the direction of bone drill guidance, medical science, surgery, etc., can solve the problems of increasing the amount of X-ray radiation of patients, prolonging the operation time, etc., and achieves the effect of easy promotion and reasonable production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
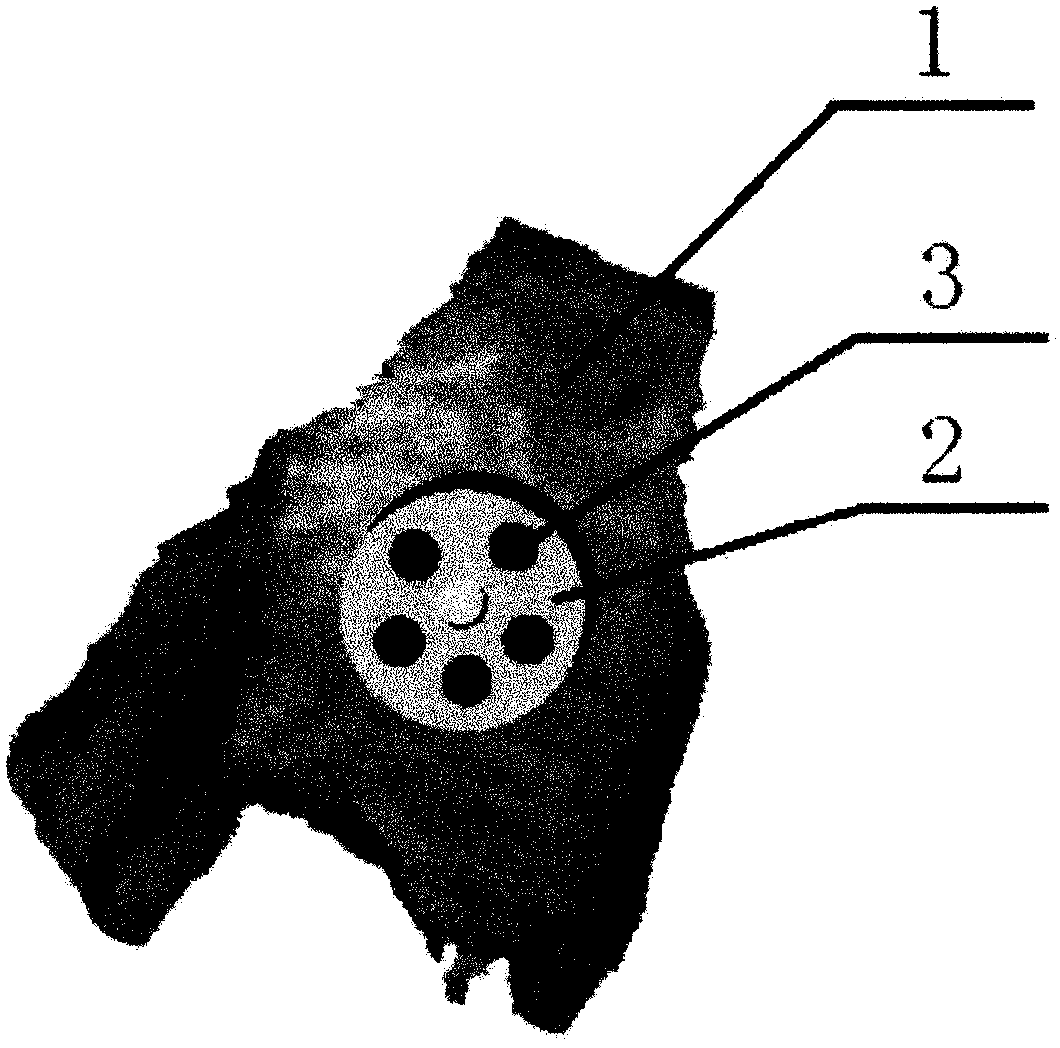
[0027] see image 3 As shown, the present invention also provides a method for preparing a three-dimensional decompression device based on the above-mentioned avascular necrosis of the femoral head, comprising the following steps:
[0028] Step S101. Import the CT three-dimensional image data (general dicom format) of the patient into MIMICS software, extract the bone data of the proximal femur on the surgical side by the MIMICS software, and calculate and generate the 3D image of the proximal femur bone;
[0029] Step S102. Create a mask of the proximal femur bone in the MIMICS software, and set an expansion value of the mask; specifically, the expansion value of the mask is 6 mm.
[0030] Step S103. Apply the generated mask to subtract the original skeleton image to obtain a new mask for skeleton shelling; specifically, the thickness of the new mask for skeleton shelling is 6 mm.
[0031]Step S104. Use MIMICS software to edit the new mask of the bone shell, only keep the bo...
specific example
[0044] 1. Determination of the anatomical parameters of the normal human proximal femur
[0045] 100 cases of hip joints without deformity were randomly selected from the CT imaging system of our hospital. The proximal femoral neck shaft angle, femoral neck diameter, femoral head diameter, and femoral neck axis length were measured by the hospital CT imaging system to determine the data distribution interval of each parameter.
[0046] 2. Design guidance system and make guidance system
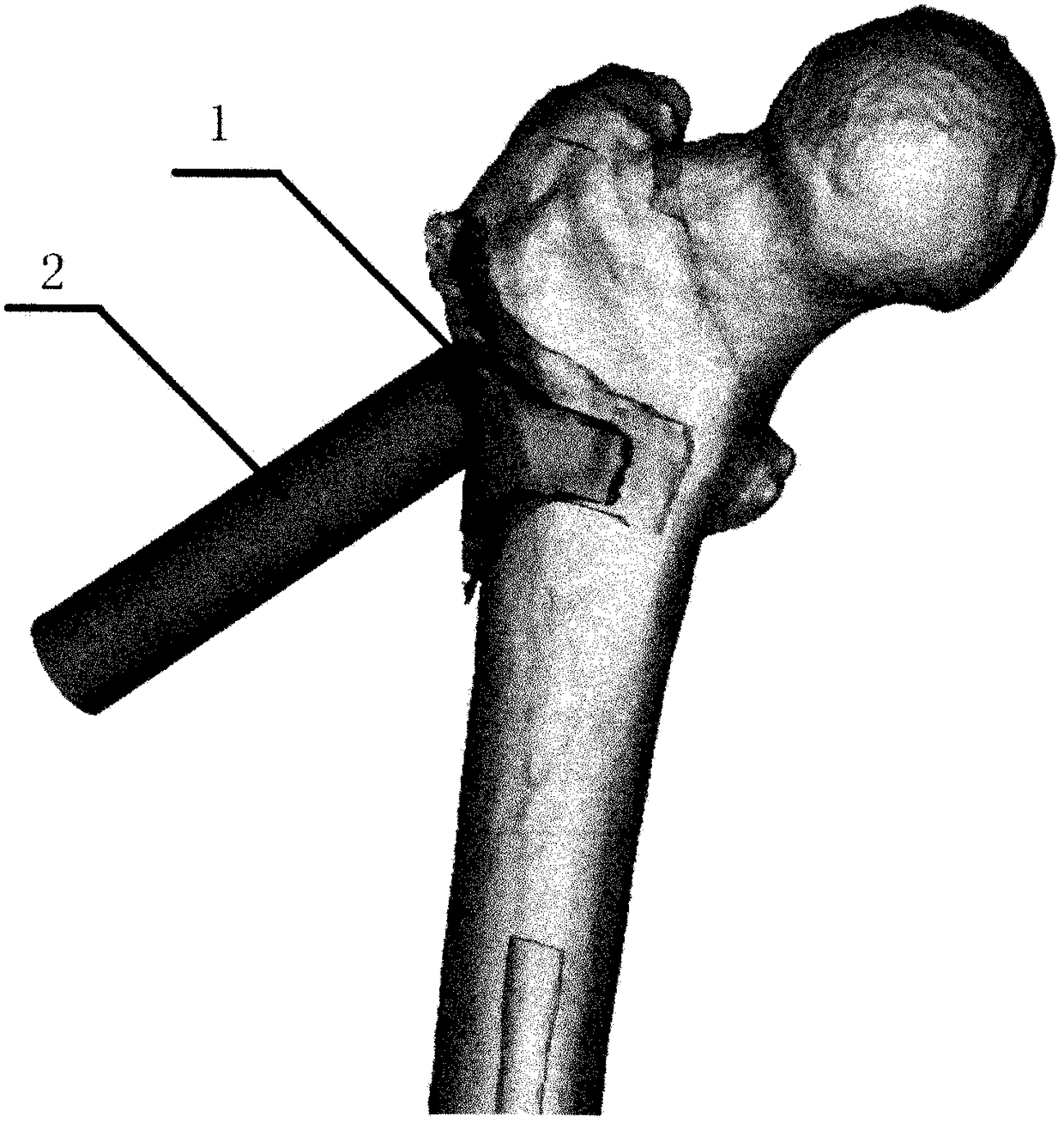
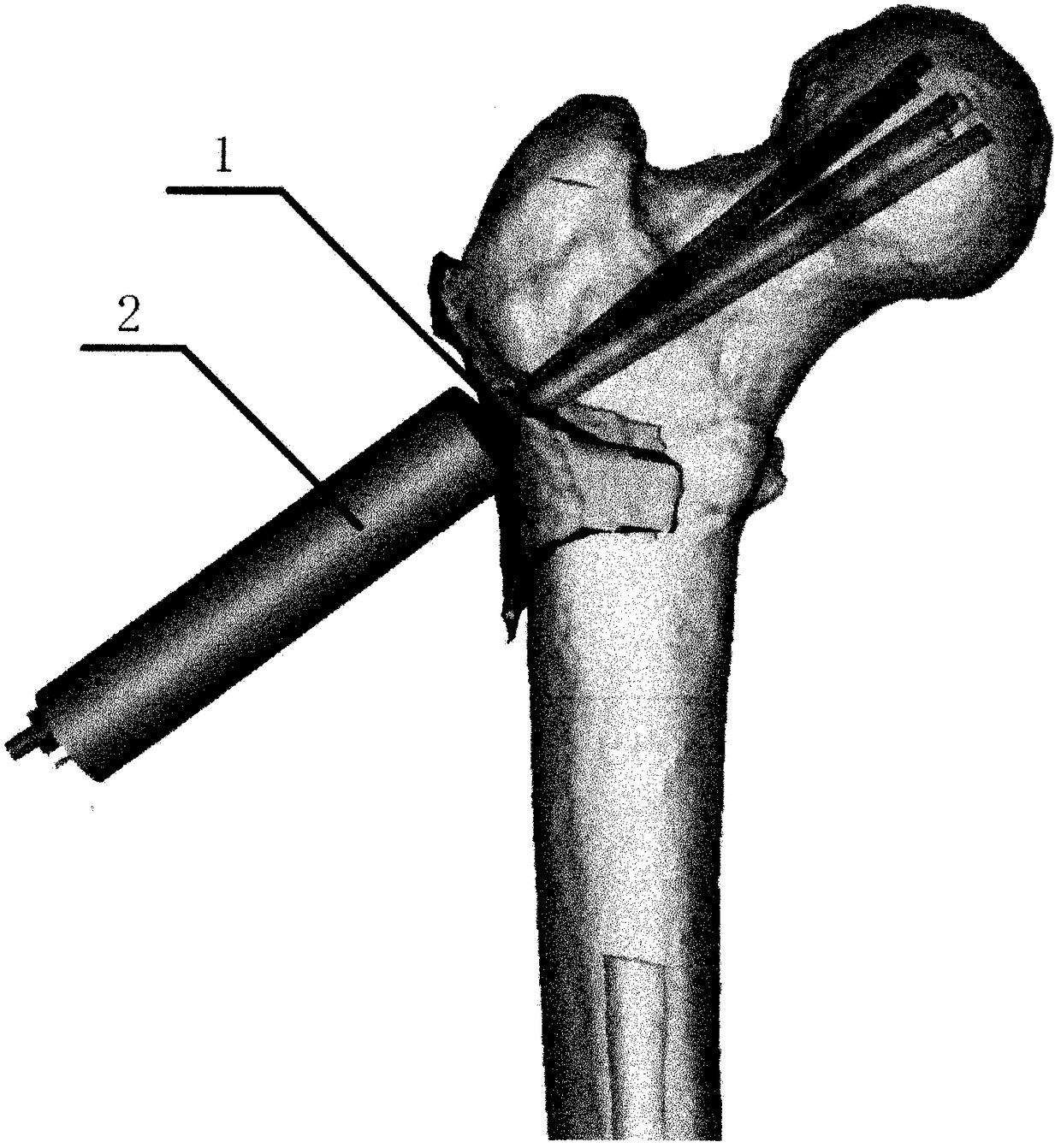
[0047] After determining the anatomical parameters of the normal proximal femur, according to the limitations of the parameters, the CDA software was used to first set up three digital samples of the guide with different arrangements of the decompression holes, and then the guide model was made by a 3D printer and tested on the normal femur model. accuracy. After confirming that the guide is working properly, the medical stainless steel metal guide and matching drill can be designed according...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com