A Porous ZrO2 Mesoscopic Crystal with Large Specific Surface
A technology of mesoscopic crystals and large specific surface, applied in catalyst carrier, inorganic chemistry, zirconia and other directions, can solve the problems of unfavorable active metal loading, low specific surface area, and no ZrO2 mesocrystals, etc., to achieve excellent catalytic performance, Good monodispersity and rich pores
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Dissolve 1.28 g of cetyltrimethylammonium bromide, 11.28 g of zirconium oxychloride octahydrate and 4.2 g of urea in 50 mL of deionized water to prepare a mixed solution, and calibrate the above mixed solution to 70 mL with deionized water Then transfer to a high-temperature reactor with a volume of 100 mL (that is, the molar concentration of zirconium oxychloride octahydrate is 0.5 mol / L, and the molar ratio of zirconium oxychloride octahydrate to cetyltrimethylammonium bromide and urea 1:0.05:2). The reaction kettle was put into a blast drying oven, the reaction temperature was controlled at 150 °C, and the reaction time was 24 h. The obtained product was washed by centrifugation to remove impurity ions and then dried at 60 °C for 8 h to obtain ZrO 2 mesoscopic crystals.
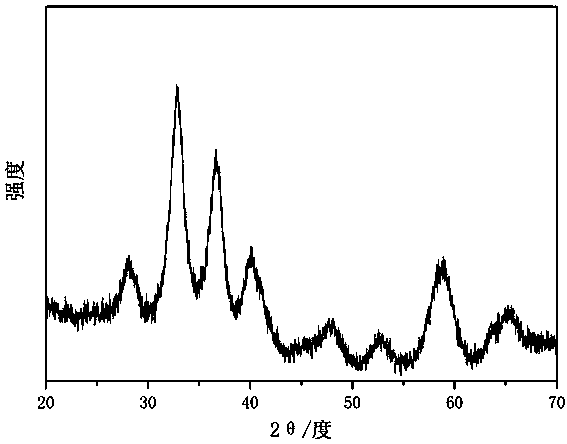
[0026] figure 1 Is the ZrO prepared in this embodiment 2 XRD patterns of mesoscopic crystals. Depend on figure 1 It can be seen that the prepared ZrO 2 Was monoclinic phase.
[0027] figur...
Embodiment 2
[0031] Dissolve 2.04 g of cetyltrimethylammonium bromide, 4.51 g of zirconium oxychloride octahydrate and 1.68 g of urea in 50 mL of deionized water to prepare a mixed solution, and calibrate the above mixed solution to 70 mL with deionized water Transfer volume to be in the high-temperature reactor of 100 mL (namely zirconium oxychloride octahydrate molar concentration is 0.2 mol / L, the molar ratio of zirconium oxychloride octahydrate and cetyltrimethylammonium bromide, urea is 1:0.4:2). The reaction kettle was put into a blast drying oven, the reaction temperature was controlled at 130 °C, and the reaction time was 48 h. The obtained product was washed by centrifugation to remove impurity ions and then dried at 60 °C for 8 h to obtain ZrO 2 mesoscopic crystals.
[0032] Figure 5 Is the ZrO prepared in this embodiment 2 SEM images of mesoscopic crystals. Figure 5 It shows that the prepared ZrO 2 The particles are also rice-like mesoscopic crystals.
[0033] N 2 -Phy...
Embodiment 3
[0035] Dissolve 2.55 g of cetyltrimethylammonium bromide, 22.56 g of zirconium oxychloride octahydrate and 8.48 g of urea in 50 mL of deionized water to prepare a mixed solution, and calibrate the above mixed solution to 70 mL with deionized water Transfer to a high-temperature reactor with a volume of 100 mL (that is, the molar concentration of zirconium oxychloride octahydrate is 1 mol / L, and the molar ratio of zirconium oxychloride octahydrate to cetyltrimethylammonium bromide and urea is 1 :0.1:2). The reaction kettle was put into a blast drying oven, the reaction temperature was controlled at 200 °C, and the reaction time was 3 h. The obtained product was washed by centrifugation to remove impurity ions, and then dried at 60 °C for 8 h to obtain ZrO 2 mesoscopic crystals.
[0036] Figure 6 Is the ZrO prepared in this embodiment 2 SEM images of mesoscopic crystals. Figure 6 It shows that the prepared ZrO 2 The particles are also rice-like mesoscopic crystals.
[0...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com