Synthesis method of N-omega-nitro-L-benzyl arginine hydrochloride
A kind of technology of arginine benzyl ester hydrochloride and synthesis method, which is applied in chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., can solve problems such as unfavorable mass production, high cost, and many steps, and reduce production And the effect of follow-up processing cost, low raw material cost, and less solvent amount
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] a. Mix 100g Boc-Arg(NO 2 )-OH (319.3 313.19nmol) suspended in 500ml benzyl alcohol;
[0017] b. Add 111.78g SOCl dropwise at 5°C 2 (118.97 939.57 nmol);
[0018] C. Insulate at 5-8°C and stir for 48 hours;
[0019] d. At 10°C, add methanol 100ml, diethyl ether 500ml, stir and crystallize, and dry to obtain crude product 108.55g H-Arg(NO 2 )-obzl.hcl;
[0020] e. The solid was dissolved by heating with 325.65ml of methanol, and crystallized by adding 976.95ml of ether after cooling;
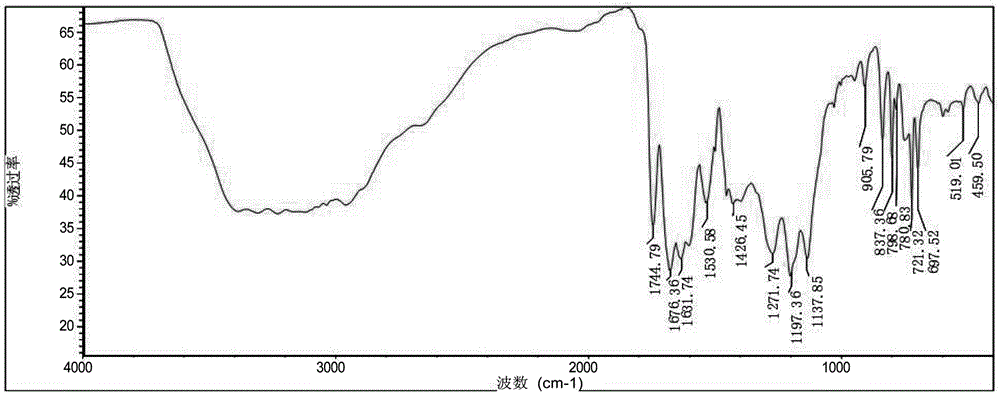
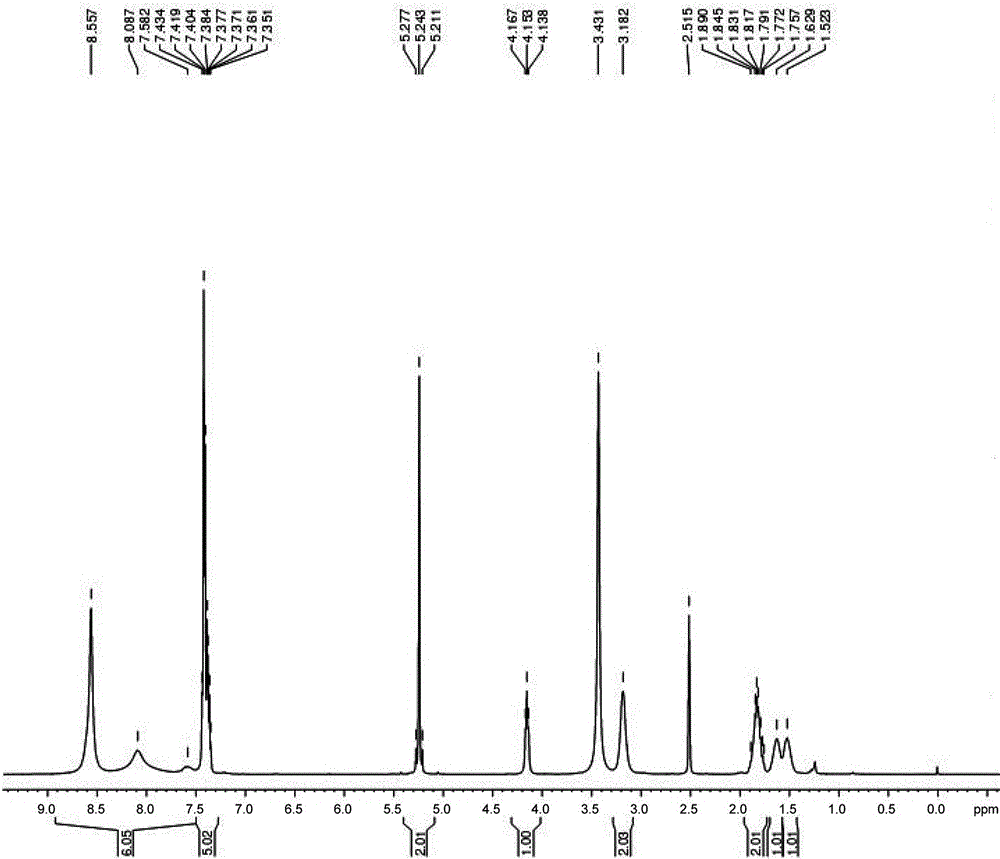
[0021] Filter and dry to get 80g fine product with a rate of 73.7%. The structure was confirmed to be correct by infrared and NMR, see figure 1 , figure 2 .
Embodiment 2
[0023] a. Mix 100g Boc-Arg(NO 2 )-OH (319.3 313.19nmol) suspended in 500ml benzyl alcohol;
[0024] b. Add 74.52g SOCl dropwise at 0°C 2 (118.97 626.38 nmol);
[0025] c. Insulate at 5-8°C and stir for 48 hours;
[0026] d. At 10°C, add 100ml of methanol and 500ml of diethyl ether to stir and crystallize, and dry to obtain 90g of crude product H-Arg(NO 2 )-obzl.hcl;
[0027] e. The solid was dissolved by heating with 270ml of methanol, and crystallized by adding 810ml of ether after cooling;
[0028] Filtration and drying to obtain 50g of fine product yield 46%. The structure was confirmed to be correct by infrared and NMR, see figure 1 , figure 2 .
Embodiment 3
[0030] a. Mix 100g Boc-Arg(NO 2 )-OH (319.3 313.19nmol) suspended in 500ml benzyl alcohol;
[0031] b. Add 111.78g SOCl dropwise at 5°C 2 (118.97 939.57 nmol);
[0032] c. Insulate at 5-8°C and stir for 24 hours;
[0033] d. At 10°C, add 100ml of methanol and 500ml of diethyl ether, stir and crystallize, and dry to obtain 95g of crude product h-arg(no2)-obzl.hcl
[0034] e. The solid is dissolved by heating with 285ml of methanol, and crystallized by adding 855ml of ether after cooling
[0035] Filtration and drying yielded 68g of fine product, yield 62.6%. The structure was confirmed to be correct by infrared and NMR, see figure 1 , figure 2 .
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap