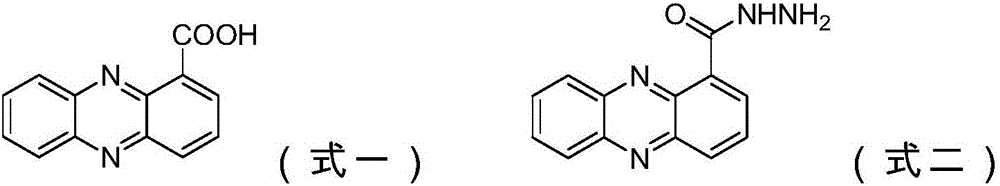
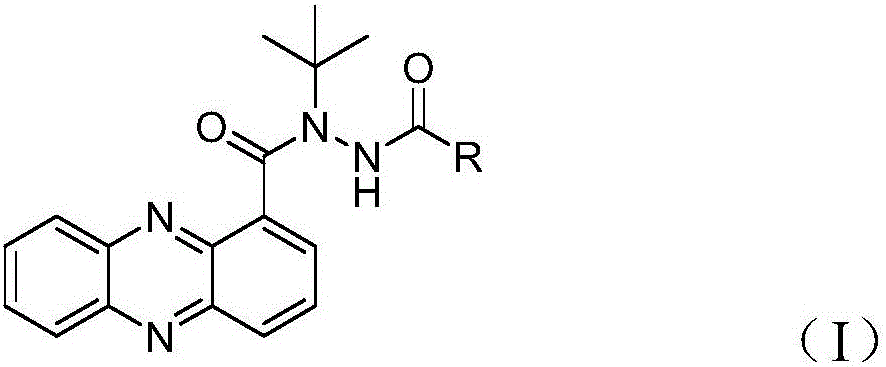
Azophenylene-1-bishydrazide carboxylate compound and bactericidal composition comprising compound
A technology of carboxylic acid dihydrazide and composition, which is applied in the field of biopesticides, can solve the problems of difficult dosage form processing and poor solubility, and achieve good bactericidal effect, high biological activity, and broad-spectrum bactericidal activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0032] The synthetic method of phenazine-1-carboxylic acid dihydrazide compound of the present invention comprises the following steps:
[0033]
[0034] Taking N-tert-butyl-N'-(2-methylbenzoyl) phenazine-1-carboxylhydrazide as an example below, set forth the synthetic method of phenazine-1-carboxylic acid dihydrazide compounds of the present invention .
Embodiment 1
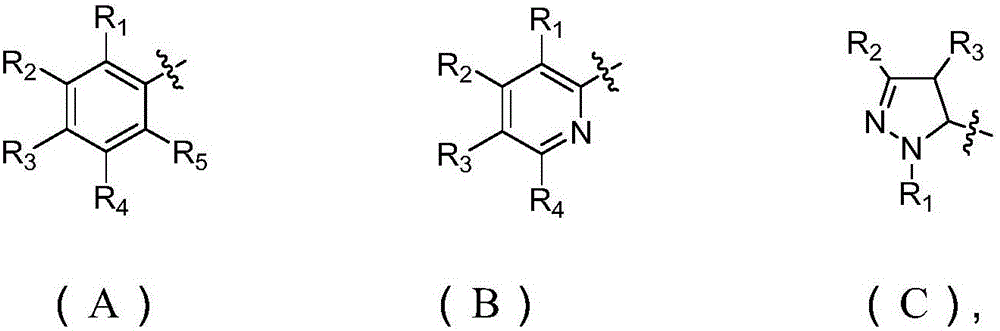
[0036] N-tert-butyl-N'-(2-methylbenzoyl)phenazine-1-carboxhydrazide (R in general formula (I) is substituted phenyl (A), R1 is CH 3 , R2, R3, R4 and R5 are H) synthetic steps are as follows:
[0037] (1) Synthesis of 2-methylphenylformyl chloride:
[0038]
[0039] Add 2.05g (15.0mmol) of 2-methylbenzoic acid into a 100mL single-port reaction flask, slowly add 15mL of thionyl chloride dropwise under stirring, then heat to reflux until the solid disappears completely, continue to reflux for 2 to 3 hours, and then Remove the solvent on a rotary evaporator, add a small amount of toluene to dissolve, and then spin dry to remove excess thionyl chloride as much as possible. Then add a certain amount of dichloromethane to dissolve and use in the next step.
[0040] (2) Synthesis of N-tert-butyl-N'-2-methylphenylcarbohydrazide:
[0041]
[0042] Add 4.20g (21.0mmol) of 20% sodium hydroxide solution into a 100mL three-necked flask, cool in an ice-water bath to 0-5°C, add 2.60g...
example 265
[0072] Example 2 65% wettable powder
[0073]
[0074]
[0075] The components are mixed together and pulverized in a pulverizer until the granules are on par.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com