Triazine quaternary ammonium salt halamine antibacterial agent and preparation method thereof, and salt-free antibacterial finishing method
A salt halamine and triazine technology, applied in the field of textile antibacterial, can solve the problems of affecting the wearability of textiles, reducing mechanical strength, bad smell and other problems, achieving excellent antibacterial performance, reducing electrostatic repulsion, and solving the effect of large-scale use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0040] One, the preparation embodiment of triazine quaternary ammonium salt halide antibacterial agent
[0041] Synthesis Example 1
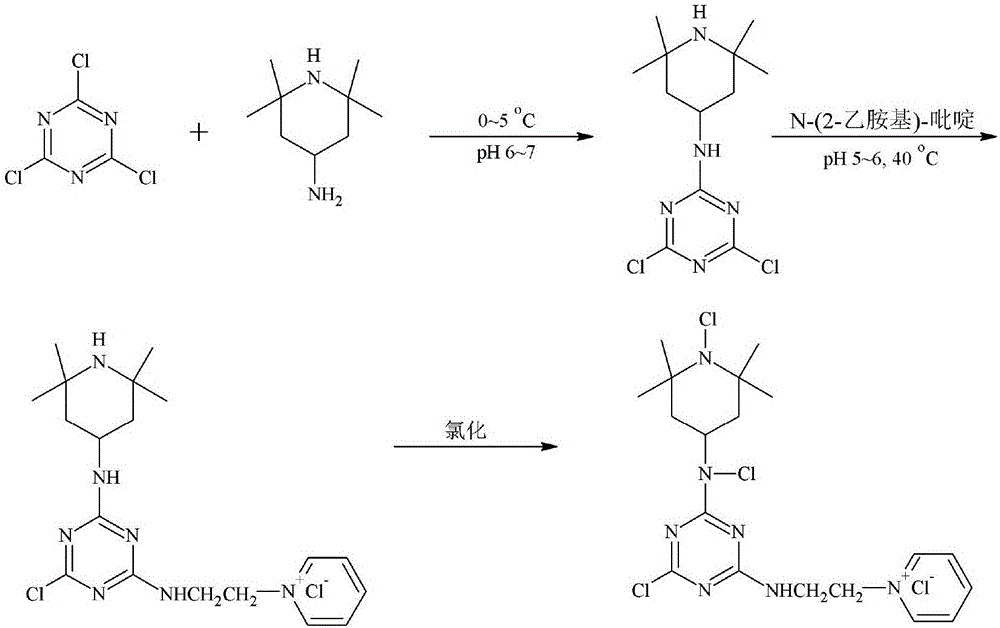
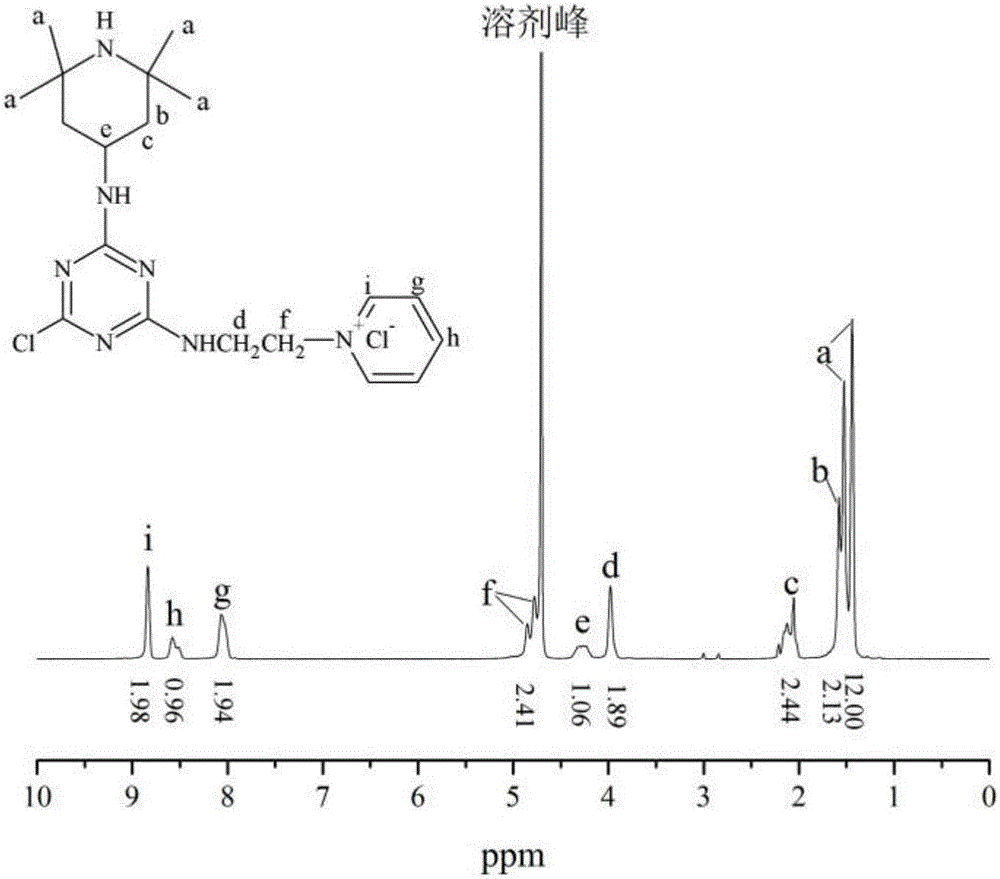
[0042] Weigh 9.22g of cyanuric chloride in a 250mL three-necked flask, add 100mL of acetone to dissolve, then slowly drop 30mL of acetone solution containing 7.80g of 2,2,6,6-tetramethylpiperidinamine into the three-necked flask, Stir in an ice bath, and react for 3 hours at a pH value of 6.0 to 7.0; then raise the temperature to 40°C, and slowly add 20 mL of an aqueous solution containing 7.9 g of N-(2-ethylamino)-pyridine dropwise to the above In the reaction system, adjust the pH value between 5.0 and 6.0 during the dropwise addition. After the dropwise addition is completed, continue to stir for 4 hours. After the reaction is completed, filter with suction, wash and dry with ethanol and acetone, and store. The above synthetic route and the NMR spectrum of the product can be found in figure 1 and figure 2 . The obtained solid product was p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com