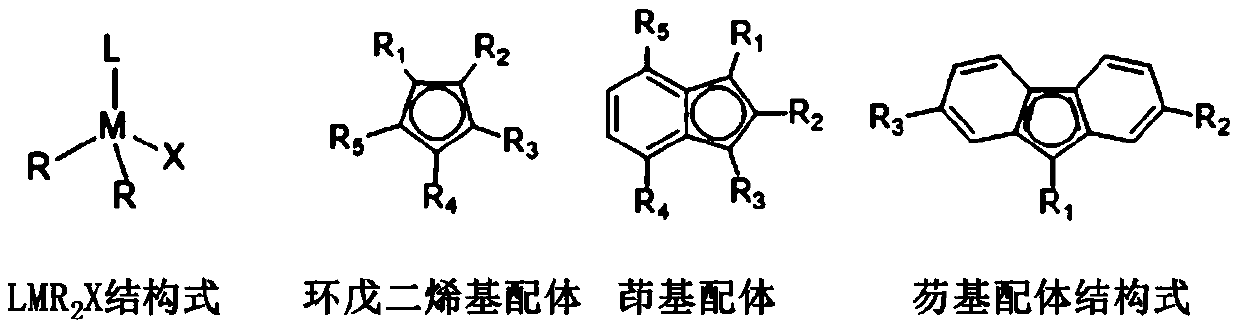
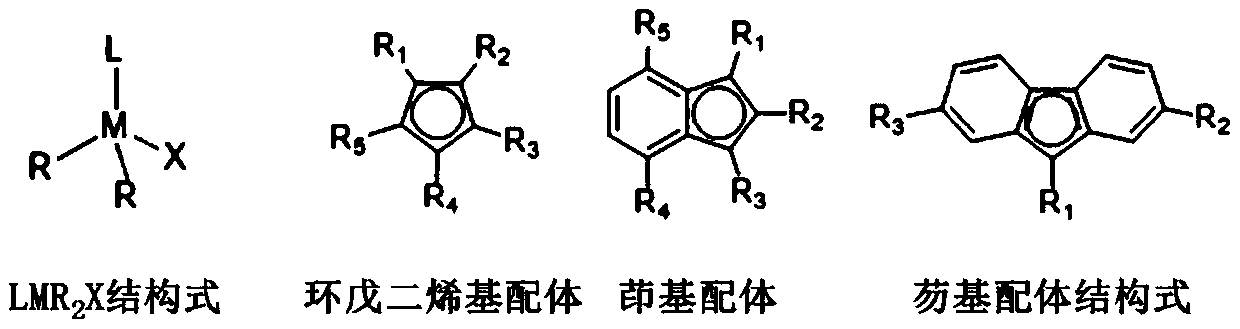
A class of rare earth catalysts containing nitrogen-containing heterocyclic carbene ligands and a method for catalyzing olefin polymerization
A nitrogen-heterocyclic carbene and rare earth catalyst technology, applied in the field of rare earth catalysts, can solve the problems of difficulty in preparing high molecular weight polymers, poor inhibition ability, and low polymerization activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0011] Example 1 Preparation of nitrogen-containing heterocyclic carbene rare earth catalyst
[0012] Different methenyl ligands and different azacyclic carbenes can be prepared by the following methods:
[0013] (1) In the glove box, weigh ScCl 3 (15mmol) was put into a Schlenk bottle filled with a magnetic stirrer, and 50mL of tetrahydrofuran was added. After sealing the Schlenk bottle, the Schlenk bottle was taken out of the glove box and stirred overnight at 80°C. Activated ScCl 3 (THF) 3 The white suspension was taken into the glove box, and the LiCH 2 SiMe 3 (45mmol) was dissolved in 15mL tetrahydrofuran, and slowly added dropwise to ScCl 3 (THF) 3 In the white suspension, react for 30min. Then, remove the solvent THF under reduced pressure, add 60mL of n-hexane for extraction, freeze the extract to remove by-products and filter while cold, and finally remove the n-hexane in the filtrate to obtain a white powder Sc(CH 2 SiMe 3 ) 3 (THF) 2 .
[0014] (2) In t...
Embodiment 2
[0017] Example 2 Preparation of polypropylene
[0018] In a glove box under the protection of inert gas nitrogen, add 20ml of toluene solution to a 100ml stainless steel reaction kettle, use a constant temperature bath to control the polymerization temperature, the polymerization temperature is -30°C, add propylene, the pressure of propylene is maintained at 0.1MPa, turn on the stirring, Add a rare earth catalyst, adopt the method provided in Example 1 to prepare a rare earth catalyst, and select 1,3-bis(trimethylsilyl)indenyl ligand (1,3-(Me 3 Si) 2 C 9 h 5 ), the nitrogen heterocyclic carbene ligand is selected from 1,3-diisopropyl-imidazolyl carbene, the amount of rare earth catalyst Sc is 2umol, the rare earth catalyst Sc and organoboron reagent [Ph 3 C][B(C 6 f 5 ) 4 ] molar ratio [Sc] / [B] is 1, after 30 minutes of polymerization reaction, methanol is added to terminate the reaction, the product is post-treated, vacuum-dried, analysis test: the number average molecu...
Embodiment 3
[0019] Example 3 Preparation of polypropylene
[0020] The polymerization reaction temperature is -20 ℃, and other polymerization reaction conditions are identical with embodiment 2, analysis test: the number average molecular weight of polypropylene is 123.9 * 10 4 g / mol, molecular weight distribution HI is 1.49.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight distribution | aaaaa | aaaaa |
| molecular weight distribution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com