Catalytic system for preparing polyether-containing polymers and preparation method of various polyether-containing polymers
A technology of polyether polythiocarbonate and polyether, which is applied in the field of polymer material synthesis and can solve the problems of little industrial application value and low catalytic efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
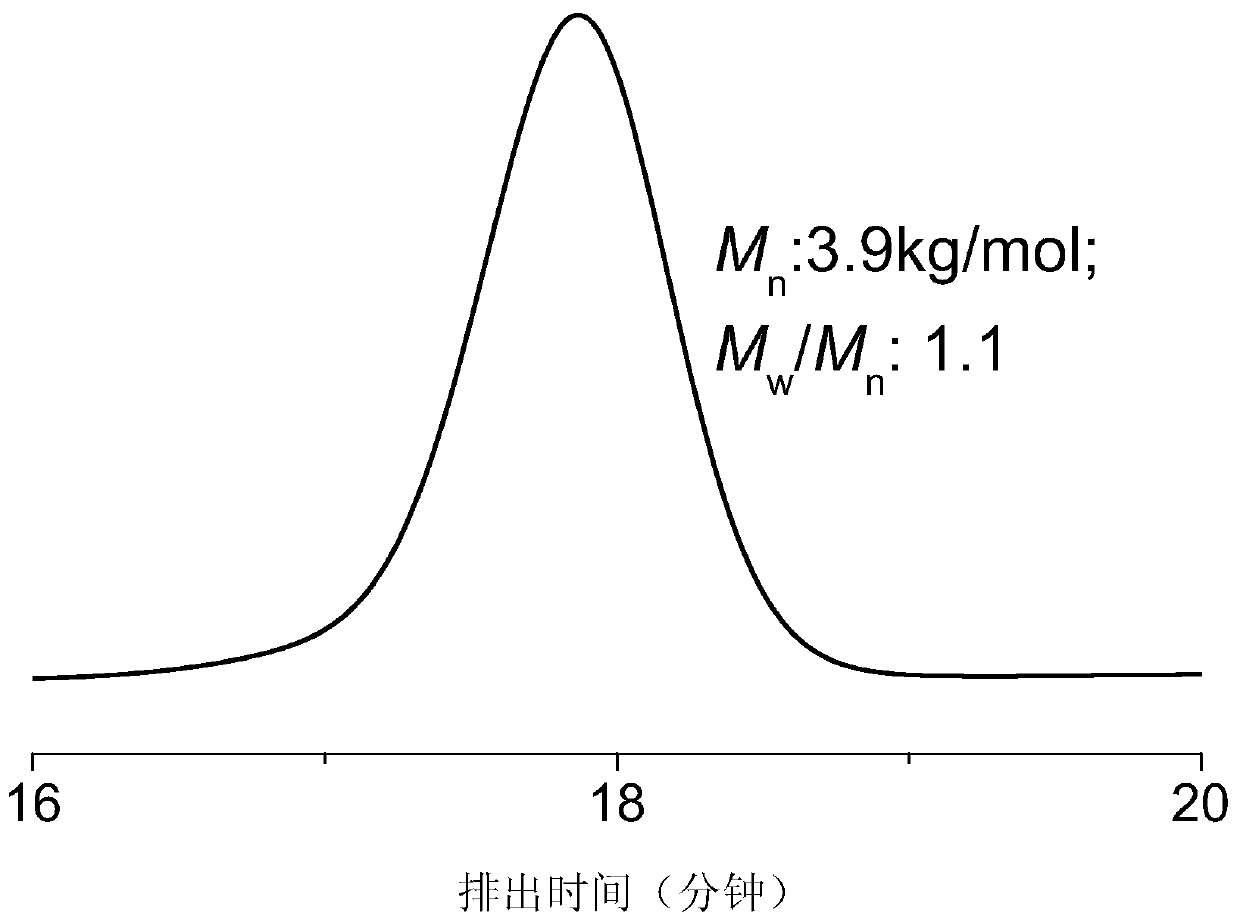
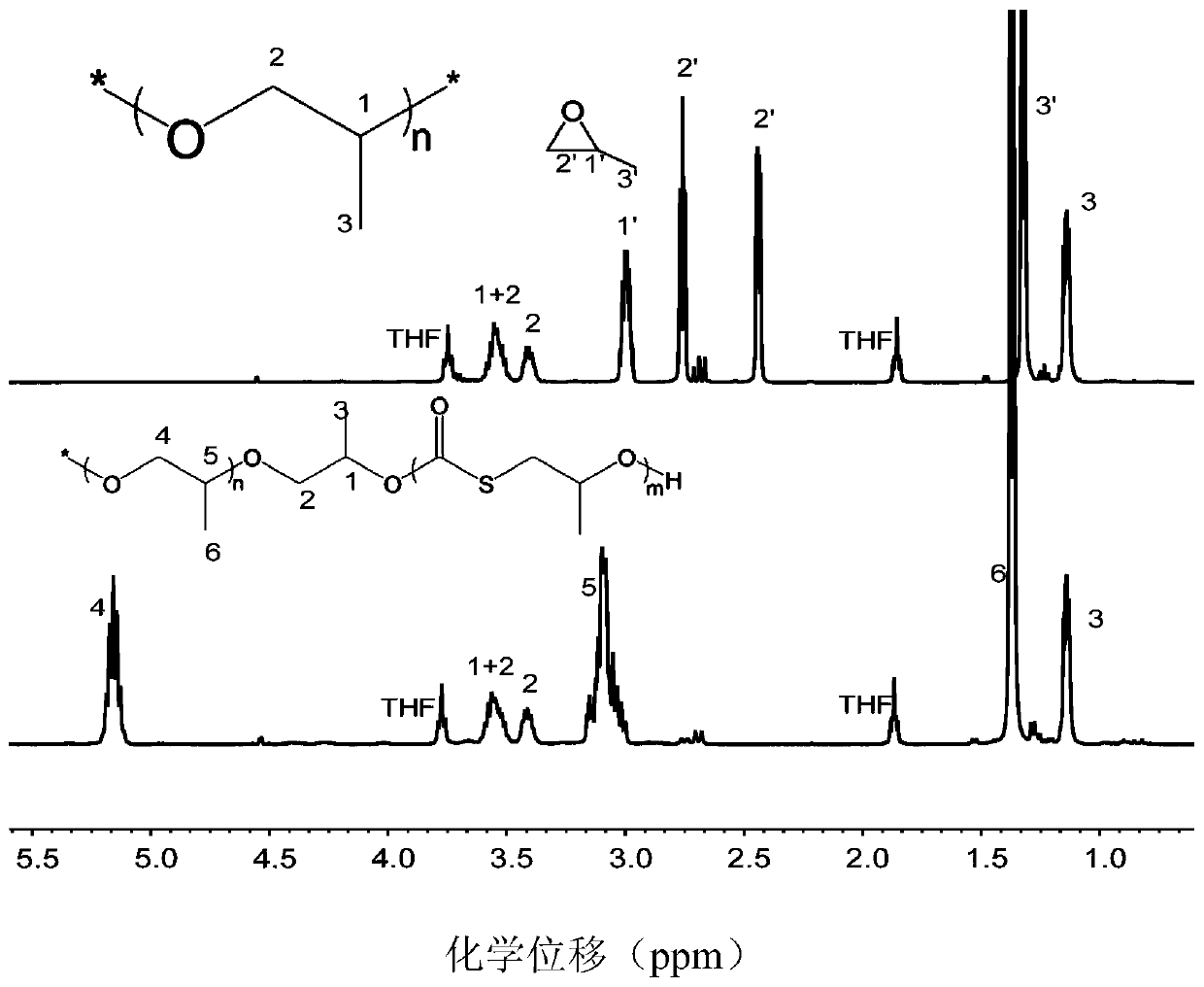
[0070] Example 1 PO homopolymerization
[0071] Before the polymerization reaction, put the 10mL Shrek tube at 110°C for about 2 hours to remove water and cool it to room temperature in a desiccator; add several masses of Lewis base 1 1-tert-butyl-2,2, 4,4,4-Penta(dimethylamino)-2Λ5,4Λ5-di(phosphorus nitrogen compound) (pKa value in acetonitrile is 33.5), methanol and triethylboron; then add 1mL has cooled to - Propylene oxide (PO) at 10°C. The molar ratio of propylene oxide, Lewis base, initiator to triethyl boron is 250 / 1 / 5 / 2. Placed in a low-temperature reactor at -10°C for 0.2h under autogenous pressure. After the reaction was completed, the crude product was first dissolved in tetrahydrofuran, and then the polymer was precipitated in a mixture of 100 mL of methanol / hydrochloric acid (the molar concentration of HCl was 5%), washed three times, and dried in vacuo to constant weight. The molecular weight and molecular weight distribution of the polymer were determined by ...
Embodiment 2
[0073] Example 2 PO homopolymerization
[0074]Before the polymerization reaction, put the 10mL Shrek tube at 110°C for about 2 hours to remove water and cool it to room temperature in a desiccator; add several masses of Lewis base 1 1-tert-butyl-2,2, 4,4,4-Penta(dimethylamino)-2Λ5,4Λ5-di(phosphorus nitrogen), ethanol and triethylboron; then 1 mL of propylene oxide (PO) which had been cooled to 0°C was added. The molar ratio of propylene oxide, Lewis base, initiator to triethylboron is 200 / 1 / 20 / 1.1. Placed in an ice-water bath at 0°C for 2 h under autogenous pressure. After the reaction was completed, the crude product was first dissolved in tetrahydrofuran, and then the polymer was precipitated in 100 mL of methanol / hydrochloric acid mixture (the molar concentration of hydrochloric acid was 5%), washed three times repeatedly, and vacuum-dried to constant weight. The molecular weight and molecular weight distribution of the polymer were determined by gel chromatography, and ...
Embodiment 3
[0075] Example 3 PO homopolymerization
[0076] Before the polymerization reaction, put the 10mL Shrek tube at 110°C for about 2 hours to remove water and cool it to room temperature in a desiccator; add several masses of Lewis base 1 1-tert-butyl-2,2, 4,4,4-Penta(dimethylamino)-2Λ5,4Λ5-dipyrazine, benzyl alcohol and triethylboron and 1 mL THF; then 1 ml propylene oxide (PO) at 25°C was added. The molar ratio of propylene oxide, Lewis base, initiator and triethyl boron is 10000 / 1 / 1 / 10; Placed at 25°C for 12h under autogenous pressure. After the reaction was completed, the crude product was first dissolved in tetrahydrofuran, and then the polymer was precipitated in 100 mL of methanol / hydrochloric acid mixture (the molar concentration of hydrochloric acid was 5%), washed three times repeatedly, and vacuum-dried to constant weight. The molecular weight and molecular weight distribution of the polymer were determined by gel chromatography, and the test results are shown in Tabl...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight distribution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com