Non-invasive marker and kit for diagnosis of lung squamous cell carcinoma patients among non-smoking or mild-smoking people
A non-invasive, non-smoking technology, applied to the determination/inspection of microorganisms, biochemical equipment and methods, DNA/RNA fragments, etc., which can solve the problems of unable to cover the population
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Example 1 Discovery and quantitative confirmation of plasma differential microRNA based on microRNA chip
[0030] 1. Experimental materials
[0031] 1. Instrument reagents
[0032] High-speed centrifuges, high-speed refrigerated centrifuges, and UV spectrophotometers were purchased from Eppendorf, Germany; NanoDrop 1000 spectrophotometers were purchased from Thermo Scientific, USA; Agilent2100Bioanalyzer System was purchased from Agilent, USA; ordinary PCR reaction instruments and real-time Fluorescence PCR instrument was purchased from American PE Company; DYCP-31B electrophoresis instrument was purchased from Beijing Liuyi Biotechnology Co., Ltd.; BIO-RAD gel imaging analysis was purchased from American Bio-Rad; ultra-low temperature refrigerator was purchased from Haier Group. mirvana TM paris TM The kit was purchased from Ambion Company of the United States; Agilent RNA 6000Pico Kit was purchased from Agilent Company of the United States; Trizol Total RNA Extra...
Embodiment 2
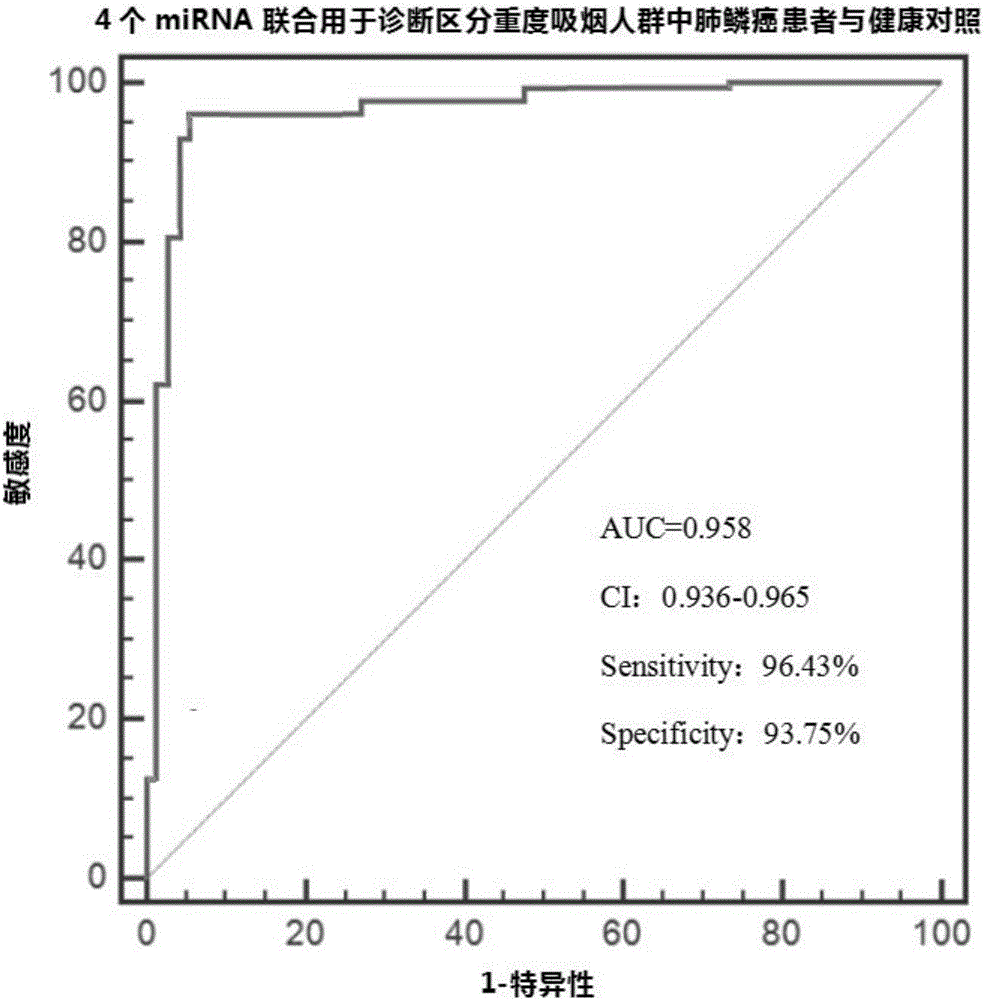
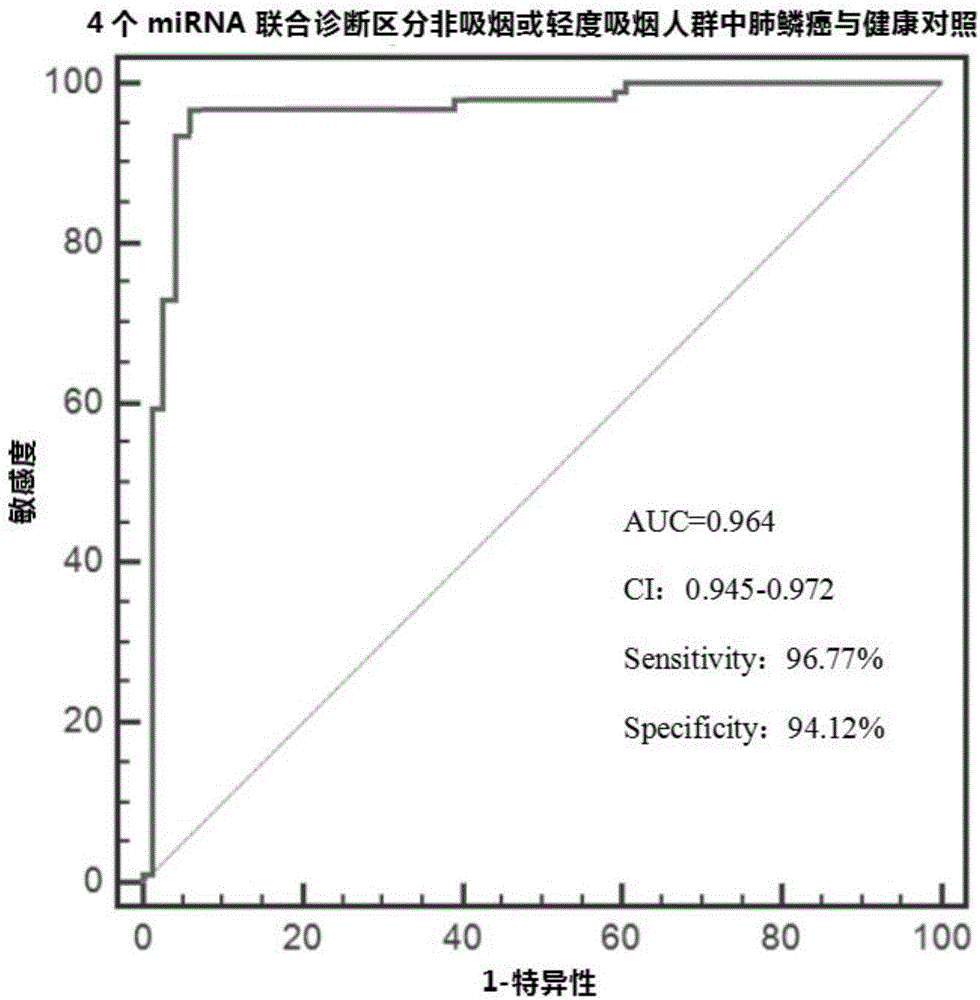
[0059] Example 2: ROC curve evaluation of diagnostic efficiency of plasma differential microRNA
[0060] The principle of ROC curve evaluation method:
[0061]The basic evaluation indicators of diagnostic tests include sensitivity, specificity, etc., and the comprehensive evaluation indicators include Youden index, ROC, AUC, etc. For the evaluation of diagnostic tests, one should first know the true category of the subjects, that is, which ones belong to the healthy group and which ones belong to the disease group. The standard for dividing the healthy group and the disease group is the gold standard (such as the histopathological diagnosis method recognized in this application). For the disease group and healthy group determined by the gold standard, the results of diagnostic tests can be divided into the following situations:
[0062] Positive (True Positive, TP); the diagnostic test is positive (consistent with the gold standard result);
[0063] Negative (True Negative,...
Embodiment 3
[0084] Example 3: Further verifying the accuracy of combined diagnosis of plasma differential microRNAs in the verification set
[0085] 1. Experimental materials
[0086] With embodiment 1.
[0087] 2. Experimental methods and results
[0088] 1. The extraction of plasma total RNA and the method of real-time fluorescent quantitative PCR are the same as in Example 1.
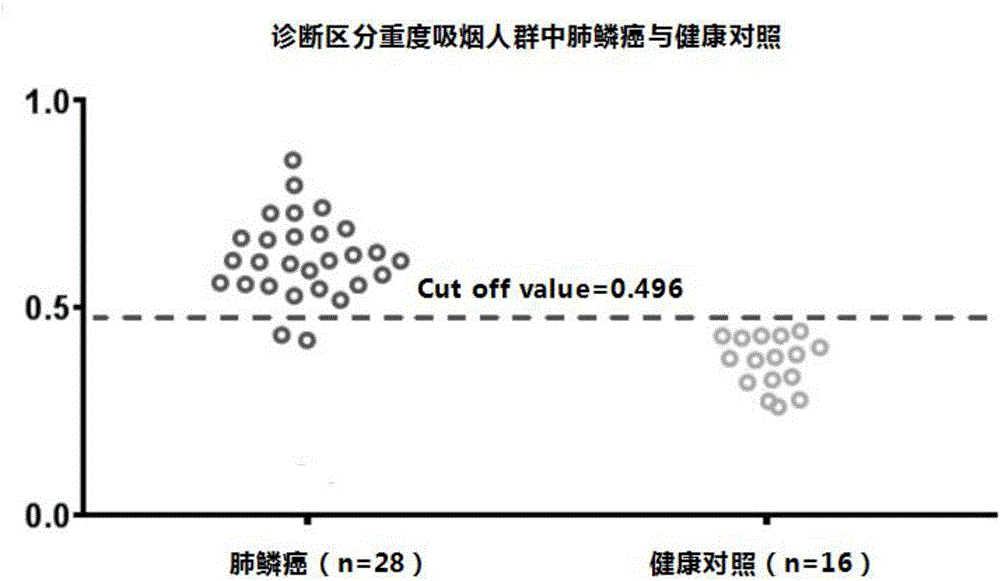
[0089] 2. In the verification set, based on the above binary logistic regression equation, the 4 plasma differential microRNAs (miR-432-3p, miR-371a-3p, miR-588, miR-676-5p) in the samples of the heavy smoking population in the verification set The standardized value of the content was transformed by binary logistic regression, and the logistic regression value of the four plasma differential microRNAs contents in the samples of heavy smoking population was calculated. Those who are lower than the optimal cut-off value of 0.496 are predicted to be healthy controls, and those who are higher than the optimal cu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com