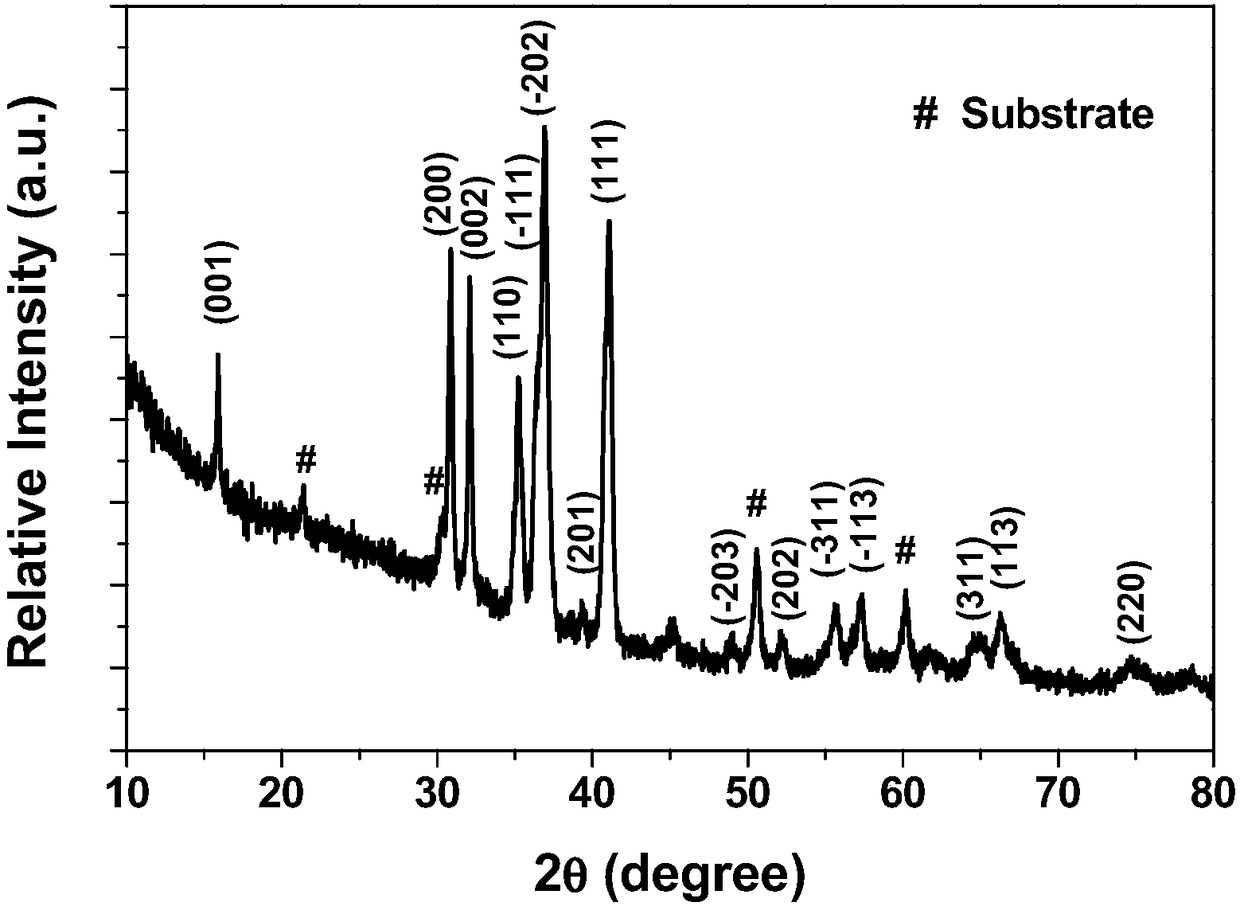
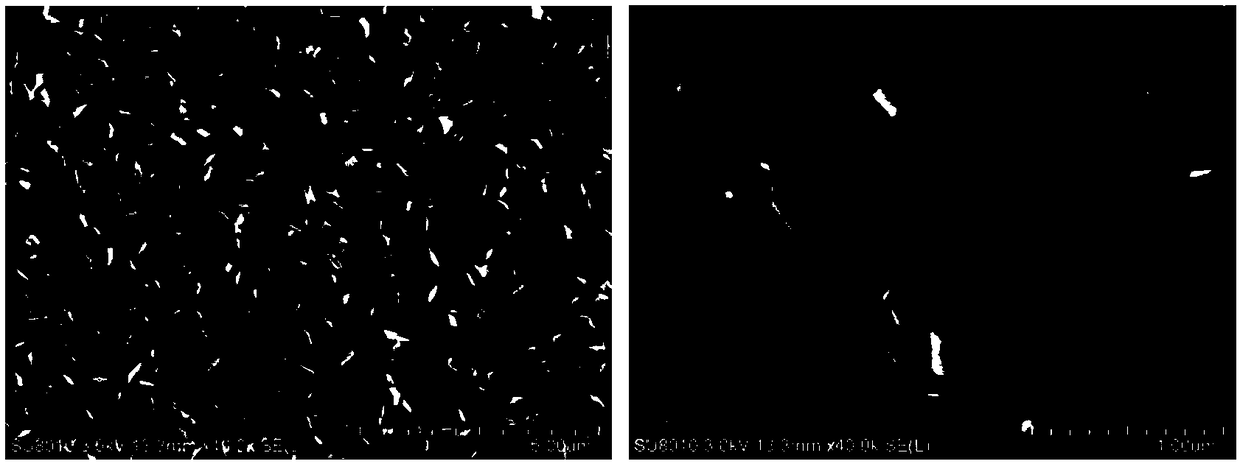
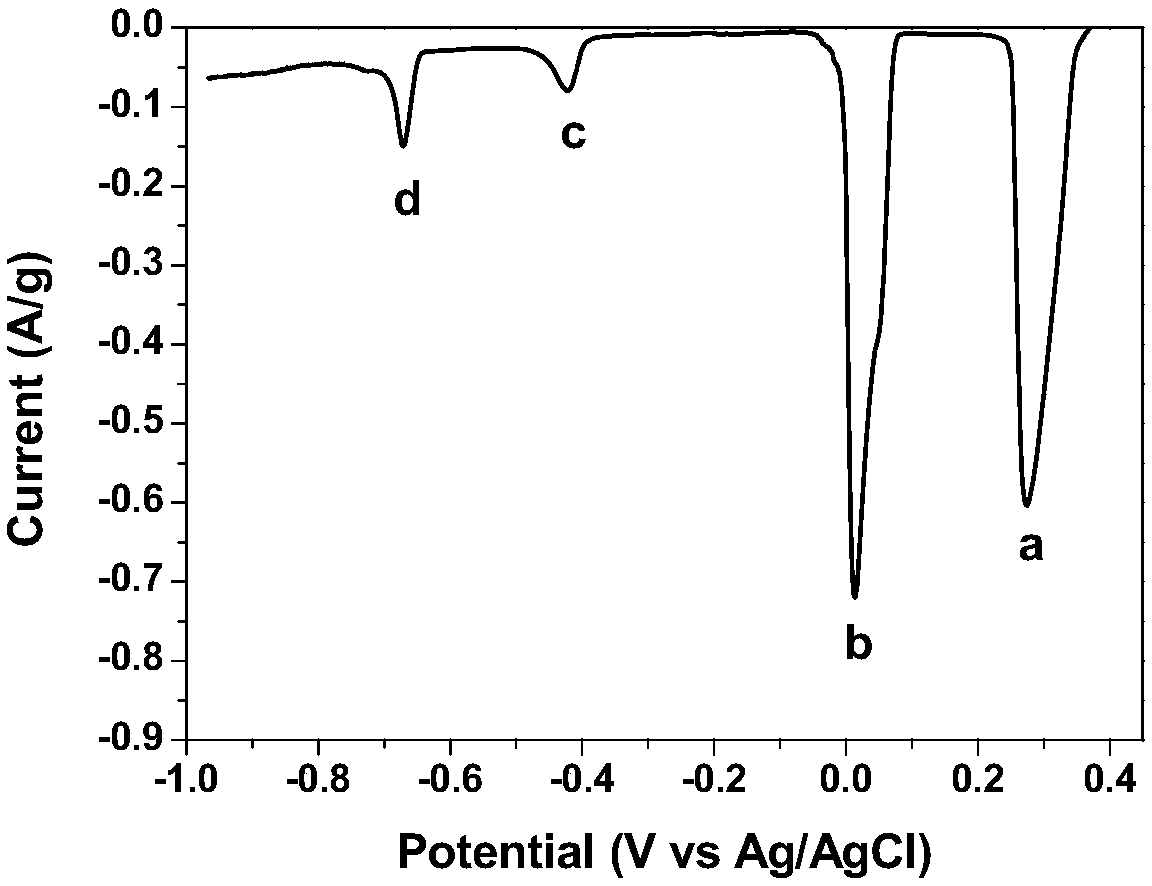
A method for preparing cathode material agcuo2 by anodic oxidation electrodeposition
A cathode material, anodizing technology, applied in electrolytic inorganic material coating, circuit, electrical components and other directions, to achieve the effect of low cost, simple and fast operation, simple experimental equipment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] 1) The working electrode, that is, the activation of ITO conductive glass: wash the ITO conductive glass with acetone for 3 times, then clean the conductive glass with deionized water in an ultrasonic cleaner for 30 minutes, and then place the conductive glass in dilute nitric acid solution for 30 seconds. , and finally washed with deionized water and set aside;
[0016] 2)AgCuO 2 Preparation of thin film electrodes: 0.000375mol of AgNO 3 and 0.0003mol of Cu(NO 3 ) 2 ·3H 2 O was stirred and dissolved in a certain amount of distilled water, and 0.05mol of NH was added dropwise while stirring. 3 , making it with Ag + and Cu 2+ Fully complex until the solution is dark blue and clear, then add 0.025mol NaOH solution to adjust the pH of the solution, and finally add distilled water to obtain a clear electrolyte solution with a volume of 100mL; ITO conductive glass is used as the working electrode, and the platinum sheet electrode is used as the counter electrode. The ...
Embodiment 2
[0020] 1) The working electrode, that is, the activation of ITO conductive glass: wash the ITO conductive glass with acetone for 3 times, then clean the conductive glass with deionized water in an ultrasonic cleaner for 30 minutes, and then place the conductive glass in dilute nitric acid solution for 30 seconds. , and finally washed with deionized water and set aside;
[0021] 2)AgCuO 2 Preparation of thin film electrodes: 0.000375mol of AgNO 3 and 0.0003mol of Cu(NO 3 ) 2 ·3H 2 O was stirred and dissolved in a certain amount of distilled water, and 0.05mol of NH was added dropwise while stirring. 3 , making it with Ag + and Cu 2+ Fully complex until the solution is dark blue and clear, then add 0.025mol NaOH solution to adjust the pH of the solution, and finally add distilled water to obtain a clear electrolyte solution with a volume of 100mL; ITO conductive glass is used as the working electrode, and the platinum sheet electrode is used as the counter electrode. The ...
Embodiment 3
[0025] 1) The working electrode, that is, the activation of ITO conductive glass: wash the ITO conductive glass with acetone for 3 times, then clean the conductive glass with deionized water in an ultrasonic cleaner for 30 minutes, and then place the conductive glass in dilute nitric acid solution for 30 seconds. , and finally washed with deionized water and set aside;
[0026] 2)AgCuO 2 Preparation of thin film electrodes: 0.000375mol of AgNO 3 and 0.0003mol of Cu(NO 3 ) 2 ·3H 2 O was stirred and dissolved in a certain amount of distilled water, and 0.05mol of NH was added dropwise while stirring. 3 , making it with Ag + and Cu 2+Fully complex until the solution is dark blue and clear, then add 0.025mol NaOH solution to adjust the pH of the solution, and finally add distilled water to obtain a clear electrolyte solution with a volume of 100mL; ITO conductive glass is used as the working electrode, and the platinum sheet electrode is used as the counter electrode. The s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com