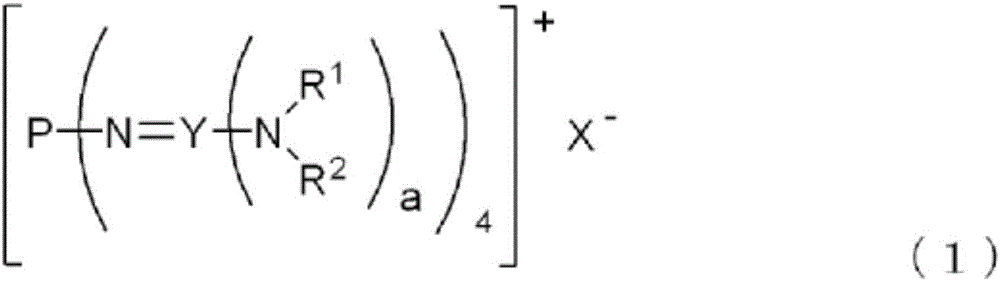Alkylene oxide polymerization catalyst and method for producing polyalkylene oxides using same
A technology of polymerization catalyst and manufacturing method, which is applied in the field of alkylene oxide polymerization catalyst and its use to manufacture polyalkylene oxide, which can solve the problems of higher unsaturation and achieve the effect of improving storage modulus and physical properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0130] Hereinafter, although an Example demonstrates this invention, this Example does not limit this invention at all.
[0131] First, the calculation method of catalytic activity and the analysis method of polyalkylene oxide will be described.
[0132] (1) Catalytic activity (unit: g / mol min)
[0133] The amount of alkylene oxide that has been reacted is marked as a (unit: g), the amount of phosphazene salt used is marked as b (unit: mol), and the time required for polymerization is marked as c (unit: min). to calculate the catalytic activity.
[0134] Catalytic activity = a / (b x c).
[0135] (2) Molecular weight of polyalkylene oxide (unit: g / mol)
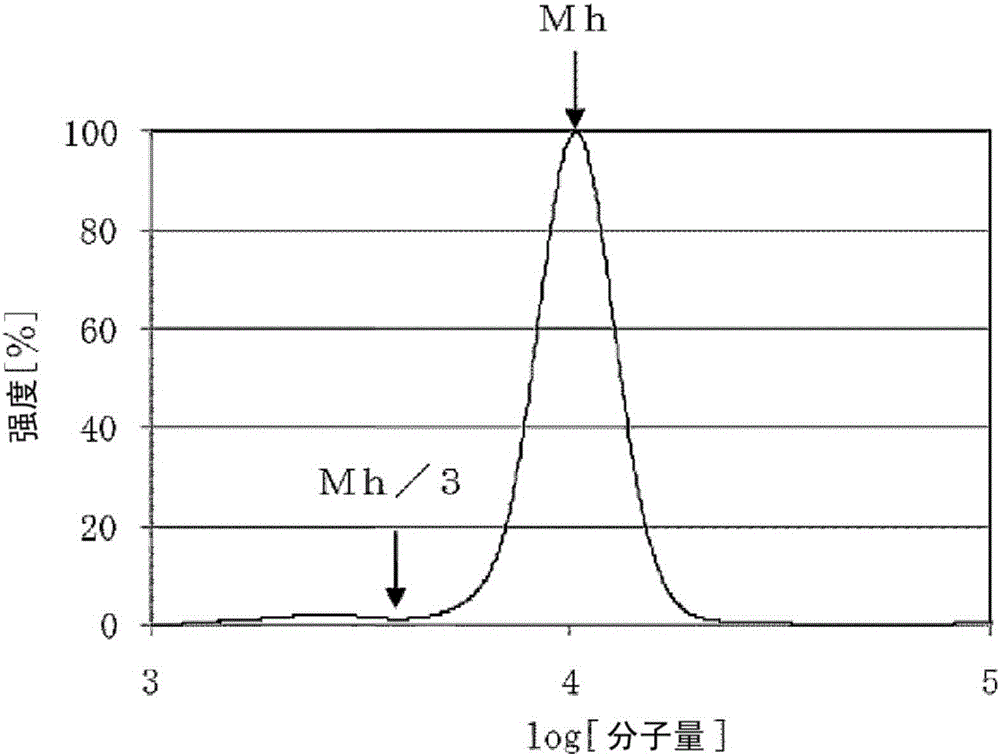
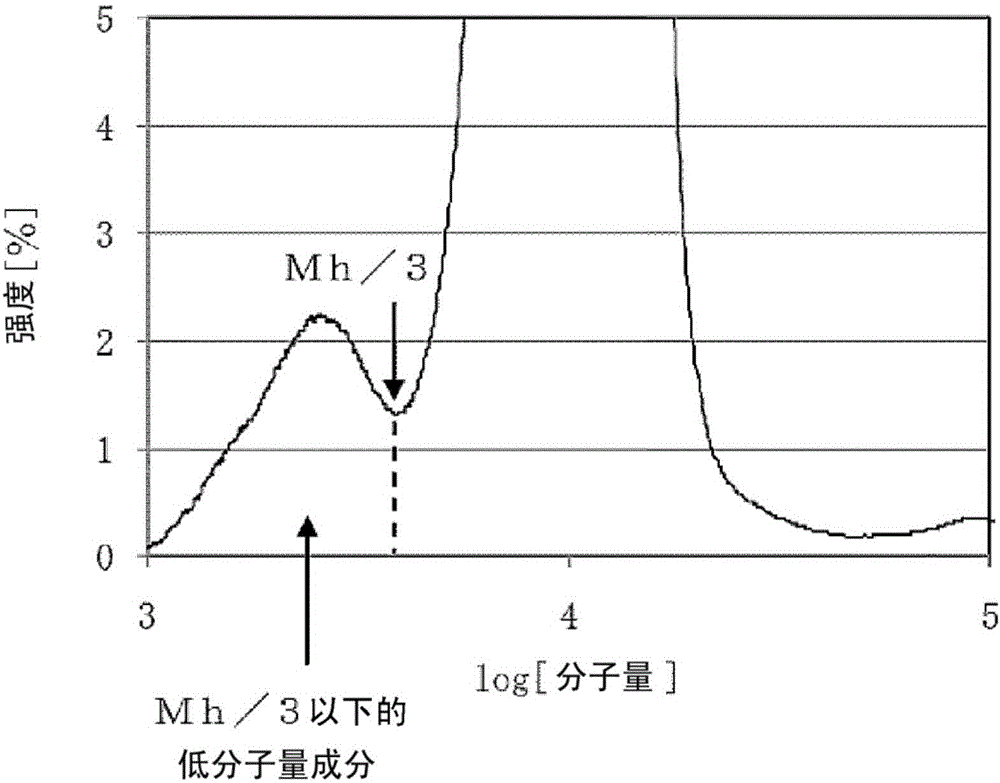
[0136] The hydroxyl value d (unit: mgKOH / g) of polyalkylene oxide was measured by the method described in JISK-1557. Let the number of functional groups of the obtained polyalkylene oxide be e, and calculate the molecular weight of polyalkylene oxide by the following formula.
[0137] Molecular weight = (56100 / d) x e.
[0...
Synthetic example 1
[0145] Synthesis Example 1 (Synthesis of Phosphazene Salt A)
[0146] A nitrogen atmosphere was set in a 2-liter four-necked flask with stirring blades, 96 g (0.46 mol) of phosphorus pentachloride and 800 ml of dehydrated toluene were added, and the mixture was stirred at 20°C. While maintaining stirring, 345 g (2.99 mol) of 1,1,3,3-tetramethylguanidine was added dropwise over 3 hours, then the temperature was raised to 100°C, and 1,1,3,3- Tetramethylguanidine 107g (0.92mol). After stirring the obtained white slurry solution at 100° C. for 14 hours, it was cooled to 80° C., and 250 ml of ion-exchanged water was added thereto, followed by stirring for 30 minutes. When stirring was stopped, the slurry was completely dissolved to obtain a biphasic solution. Oil-water separation of the obtained two-phase solution was carried out, and the water phase was recovered. 100 ml of dichloromethane was added to the obtained aqueous phase, oil and water were separated, and the dichlorome...
Synthetic example 2
[0148] Synthesis Example 2 (Synthesis of Phosphazene Salt B)
[0149] A 100 ml Schlenk bottle with a magnetic rotor was made into a nitrogen atmosphere, and 5.7 g (7.4 mmol, manufactured by Aldrich) and 16 ml of 2-propanol were added to the nitrogen atmosphere. It was stirred and dissolved at 25°C. Under the condition of maintaining stirring, 0.53 g [8.1 mmol, 1.1 mol equivalent to tetrakis[tris(dimethylamino)phosphineamino]phosphonium chloride] of 85% by weight of potassium hydroxide was dissolved in 2-propane Alcohol obtained solution. After stirring at 25° C. for 5 hours, the precipitated by-product salt was removed by filtration to obtain the target phosphazene salt B [R in the above general formula (1) with a concentration of 17% by weight and a yield of 98%. 1 is methyl, R 2 is methyl, X - is a hydroxyl anion, Y is a phosphorus atom, and a corresponds to a phosphazene salt of 3] in 2-propanol solution 32.7g.
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of unsaturation | aaaaa | aaaaa |
| degree of unsaturation | aaaaa | aaaaa |
| degree of unsaturation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com