Supersaturated solid self-emulsifying preparation and preparation method thereof
A self-emulsifying and emulsifying agent technology, which is applied in the fields of pharmaceutical formulations, emulsion delivery, and active ingredients of heterocyclic compounds, etc., and can solve problems affecting drug bioavailability and drug efficacy, low apparent solubility of drugs, equilibrium solubility, thermodynamic instability, etc. problems, achieve good development and application prospects, inhibit and delay crystallization, and reduce the effect of crystallization driving force
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Example 1 Fenofibrate supersaturated solid self-emulsifying preparation
[0033] The method for constructing the fenofibrate supersaturated solid self-emulsifying preparation of this embodiment includes the following steps:
[0034] Fenofibrate (FNB) is a poorly soluble drug, the oil phase is ethyl oleate, the emulsifier is Cremophor RH40, and the co-emulsifier is Transcutol HP. The preparation method includes the following steps:
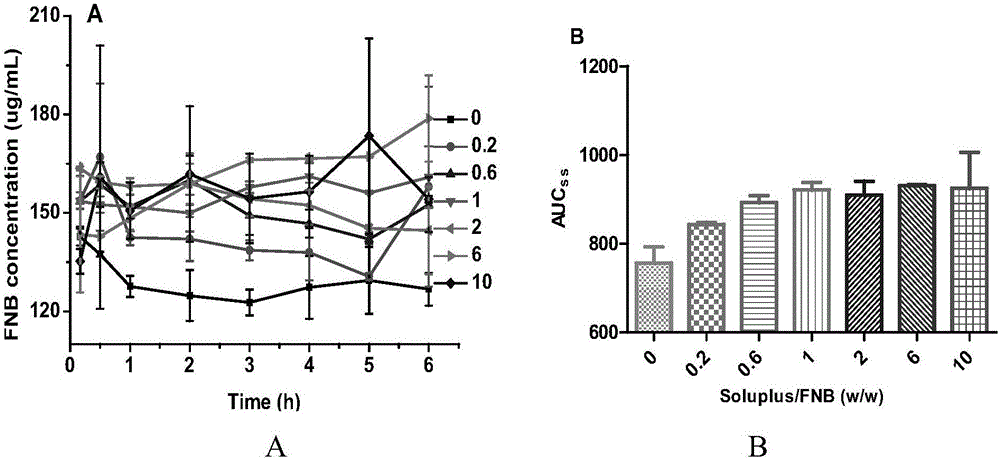
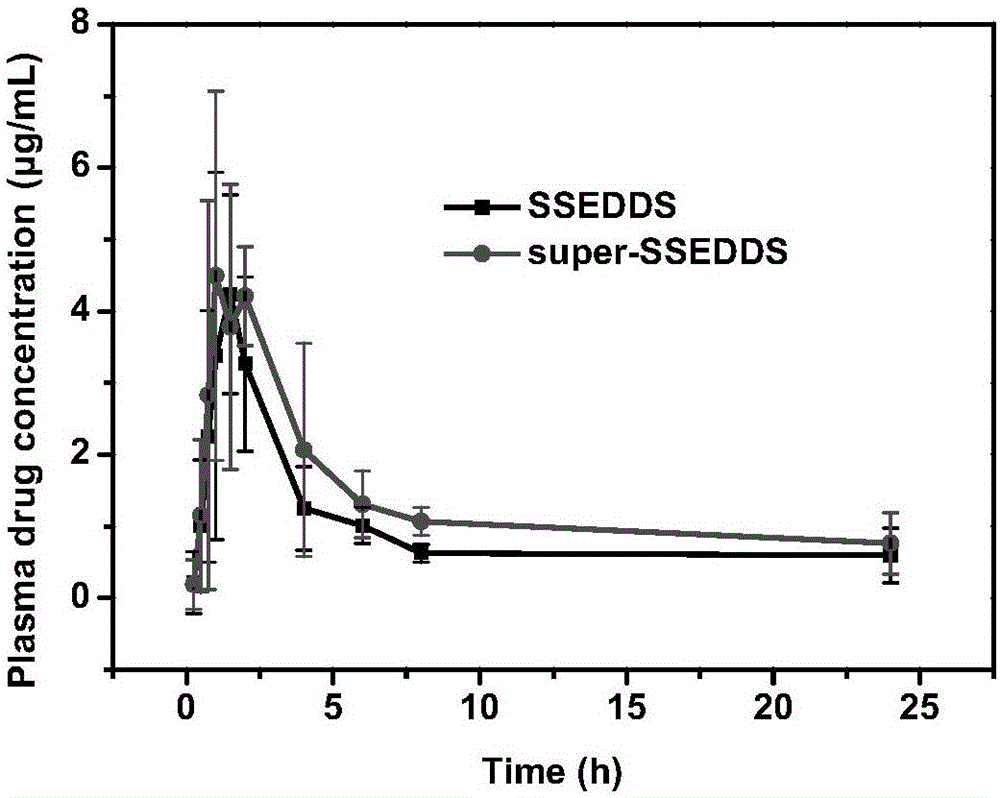
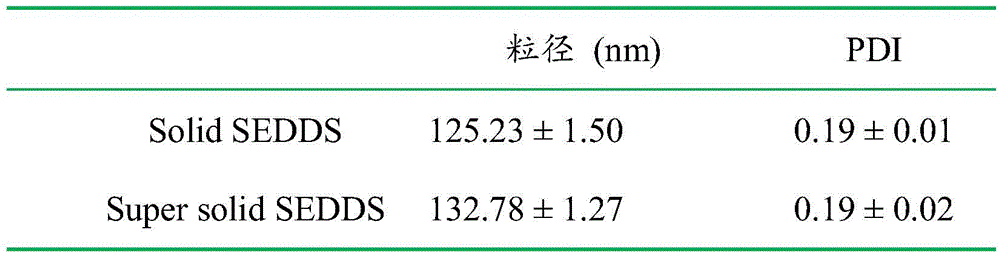
[0035] Weigh 2.55 g of the oil phase, 2.55 g of the emulsifier, and 3.4 g of the co-emulsifier to obtain a uniform and clear oily solution. Weigh 1.5 g of the poorly soluble drug FNB and add it to the above solution to dissolve to obtain the poorly soluble drug liquid SEDDS. Weigh 3.0 g of the above-mentioned poorly soluble drug liquid SEDDS, add 10 mL of absolute ethanol, add 1.0 g of solidified carrier SBA-15, and dry to remove the solvent to obtain the poorly soluble drug SEDDS. Weigh 1.0 g of the crystallization inhibitor amphiphilic polymer ...
Embodiment 2
[0036] Example 2 Celecoxib supersaturated solid self-emulsifying preparation
[0037] The method for constructing the celecoxib supersaturated solid self-emulsifying preparation of this embodiment includes the following steps:
[0038] Celecoxib is a poorly soluble drug, the oil phase is ethyl oleate, the emulsifier is Cremophor RH40, and the co-emulsifier is Transcutol HP. The preparation method includes the following steps:
[0039] Weigh 3.0g of oil phase, 3.0g of emulsifier, and 4.0g of co-emulsifier and mix to obtain a uniform and clear oily solution. Weigh 2.0g of poorly soluble drug celecoxib and add the above solution to dissolve to obtain the poorly soluble drug liquid SEDDS. Weigh 3.0 g of the above-mentioned poorly soluble drug liquid SEDDS, add 10 mL of absolute ethanol, add 1.0 g of solidified carrier SBA-15, and dry to remove the solvent to obtain the poorly soluble drug SEDDS. Weigh 0.8 g of the crystallization inhibitor amphiphilic polymer Soluplus and add it to th...
Embodiment 3
[0040] Example 3 Carbamazepine supersaturated solid self-emulsifying preparation
[0041] The method for constructing the carbamazepine supersaturated solid self-emulsifying preparation of this embodiment includes the following steps:
[0042] Carbamazepine is a poorly soluble drug, the oil phase is ethyl oleate, the emulsifier is Cremophor RH40, and the co-emulsifier is Transcutol HP. The preparation method includes the following steps:
[0043] Weigh 2.5g of oil phase, 2.5g of emulsifier, and 3.5g of co-emulsifier to obtain a uniform and clear oily solution. Weigh 1.5g of the poorly soluble drug carbamazepine and add it to the above solution to dissolve it to obtain the poorly soluble drug liquid SEDDS . Weigh 3.5 g of the above poorly soluble drug liquid SEDDS, add 10 mL of absolute ethanol, add 1.0 g of solidified carrier SBA-15, and dry to remove the solvent to obtain the poorly soluble drug SEDDS. Weigh 1.5 g of the crystallization inhibitor amphiphilic polymer Soluplus and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com