Improved anti-VEGFR (vascular endothelial growth factor receptor)-2 monoclonal antibody
A VEGFR-2, monoclonal antibody technology, applied in the direction of antibodies, antibody mimics/scaffolds, anti-tumor drugs, etc., to achieve the effects of high affinity, good biological activity, and broad development prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Example 1, Biopanning of single-chain antibody against VEGFR-2
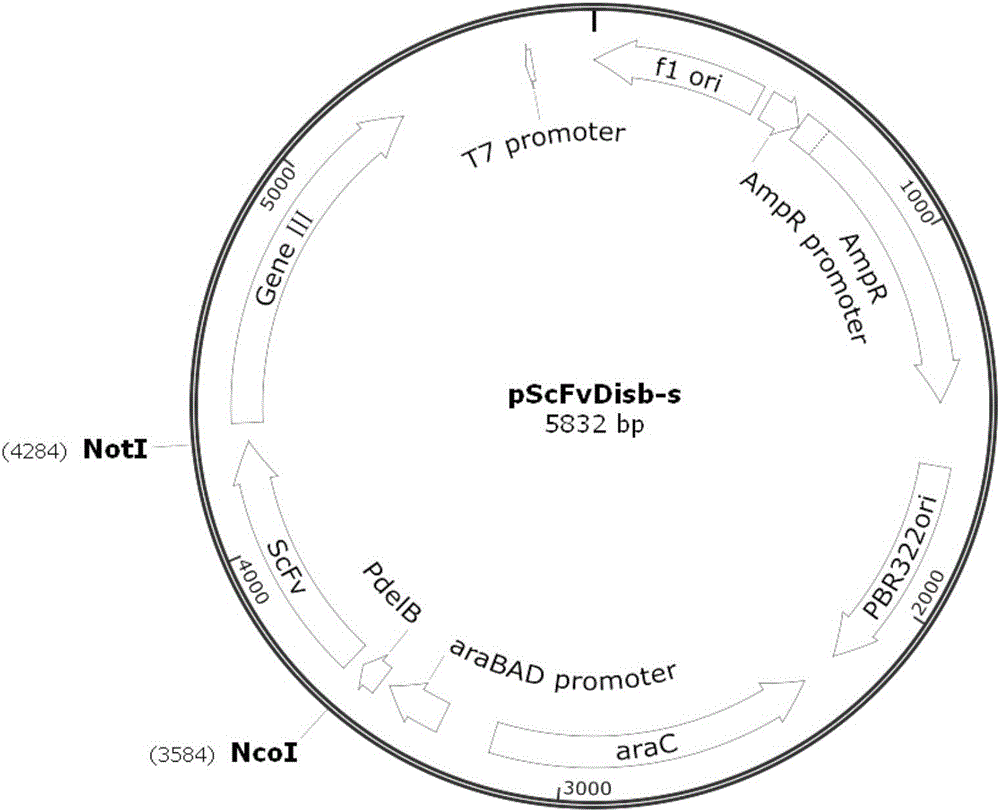
[0051] A series of gene cloning methods were used to transform the vector pCom3 vector (purchased from China Plasmid Vector Strain Cell Line Gene Collection Center) to be used for the construction and expression of phage single-chain antibody library. The transformed vector was named pScFvDisb-S, and its plasmid map is as follows: figure 1 As shown, and based on this vector, a new fully synthetic phage antibody library based on the antibody sequence in the patent CN10333247B was constructed.
[0052] The immunization tube was coated with the extracellular region of VEGFR-2 as the antigen, the amount of antigen coating was 2ug / 500ul / tube, and the tube was coated overnight at 4°C. The immunotube and the fully synthetic phage antibody library were then blocked with 4% skimmed milk powder / PBST for 1 hour at room temperature. The blocked phage antibody library was added to the immunotube for antigen-antibody ...
Embodiment 2
[0055] Example 2, Identification of Anti-VEGFR2 Phage Single-Chain Antibody Positive Clones
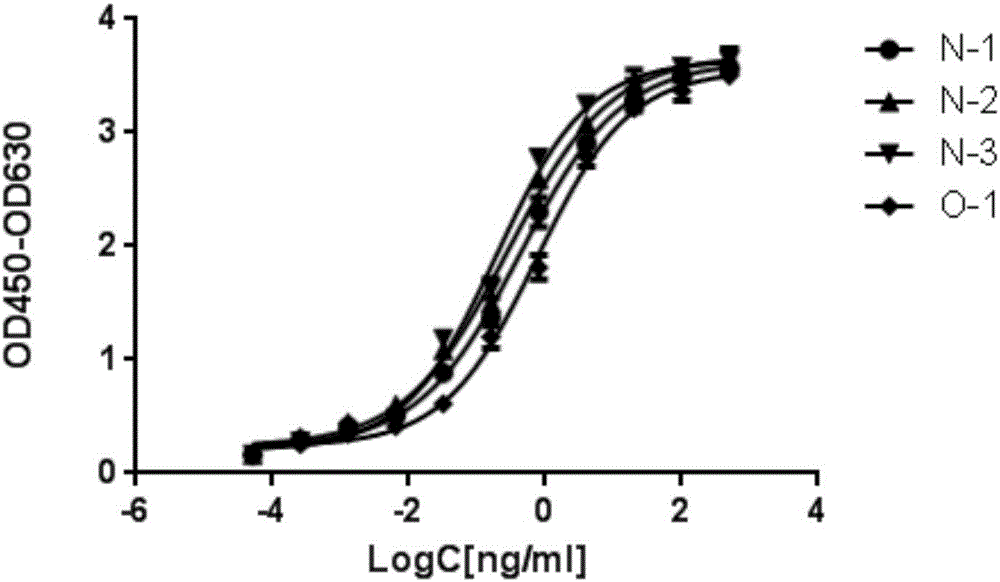
[0056] After three rounds of screening, well-separated monoclonal colonies were picked and inoculated in a 96-well deep-well plate supplemented with 2YTAG liquid medium, cultivated at 37°C and 220 rpm until the logarithmic growth phase, and about 10 10 The helper phage M13KO7 was statically infected at 37°C for 30 minutes. Centrifuge at 4000rpm at 4°C for 15 minutes, discard the supernatant, resuspend the pellet with 2YTAK, and culture overnight at 28°C at 220rpm. The supernatant containing the phage was taken for ELISA identification, and the phage of the biological antibody sequence of CN10333247B (heavy chain is CN10333247B sequence No. 3, and the light chain is CN10333247B sequence No. 9) with the same carrier and the same construction method was used as a positive control (named as O-1). Monoclonal antibodies N-1, N-2 and N-3 with higher affinity were screened, and gene sequenc...
Embodiment 3
[0057] Example 3, Gradual dilution of phage ELISA to compare the affinity of anti-VEGFR2 single-chain antibody
[0058] The clones obtained in Example 2 were displayed and purified by monoclonal phages, and the affinity was identified by phage serial dilution ELISA experiments.
[0059] Coat the extracellular region of VEGFR-2 with pH 9.6 carbonate buffer, 20ng / well / 100ul, overnight at 4°C. Washed three times with PBST, blocked with 4% milk-PBST at 37°C for 1 hour. The purified phage was diluted five-fold with 4% milk-PBST, and 100 ul of the diluted sample was added to each well, and allowed to stand at room temperature for 1 hour. The ELISA plate was washed with PBST, the HRP-anti-M13 monoclonal antibody diluted in 4% milk-PBST was added to the ELISA plate, and left at room temperature for 1 hour. TMB color development kit was used for color development at room temperature for 10 minutes. with 2M H 2 SO 4 After termination, read at 450nm / 630nm. The result is as figure...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com