Activation of the endogenous ileal brake hormone pathway for organ regeneration and related compositions, methods of treatment, diagnostics, and regulatory systems
A composition, organ technology, applied in the fields of regenerating hepatocytes, pancreatic beta cells of subjects, reducing cellular inflammation and regenerating nerve cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0403] Example 1: Formulations for Pancreas Regeneration to Improve T2D
[0404] The subject invention for the treatment of T2D relates to a pharmaceutical formulation or dosage form comprising a first active drug comprising an ileal brake hormone releasing substance, which is coated with an immediate or delayed release layer of a second active drug, said second active drug The medicament preferably comprises the antihyperglycemic drug metformin or a pharmaceutically acceptable salt thereof, or sitagliptin or a substitute from the list of available DPP-IV inhibitors as defined herein. The ileal brake hormone releasing substance is delivered in a controlled release manner from a tablet core, preferably an osmotic tablet core without gelling or swelling polymers.
[0405] The composition of the tablet core should include an ileal brake hormone releasing substance and at least one pharmaceutically acceptable excipient. In one embodiment of the invention, the tablet core comprise...
Embodiment 2
[0461] Example 2: Using MetaBrake TM pancreatic beta cell regeneration
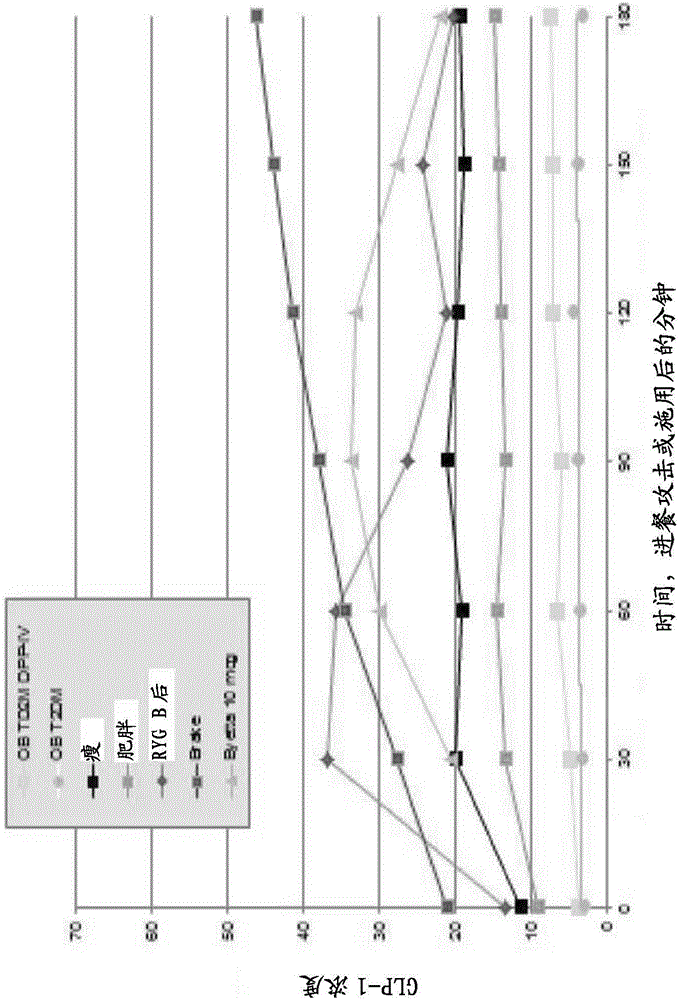
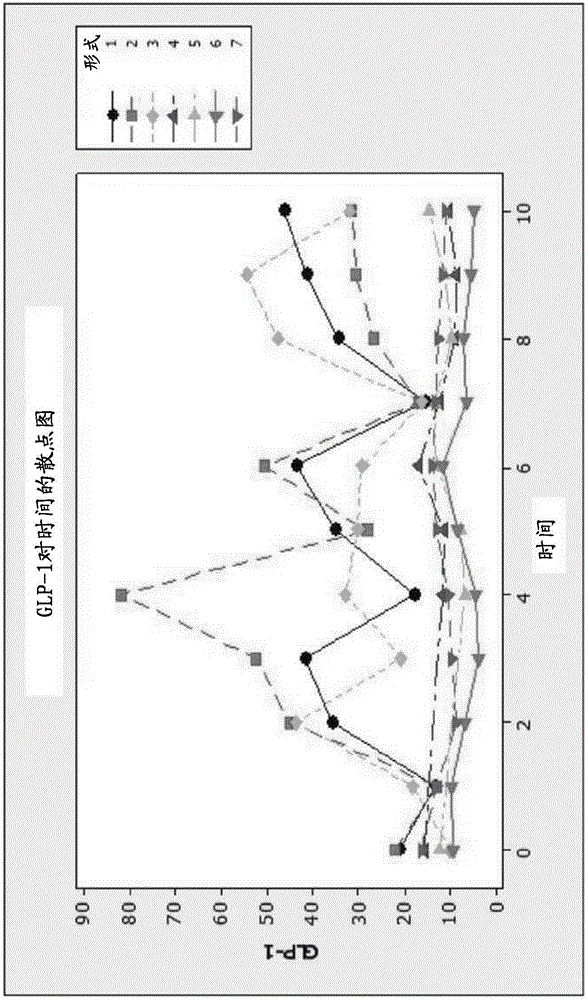
[0462] Metformin is the main treatment for T2D worldwide, and all biguanides show dose-related reductions in hyperglycemia. Several studies of metformin in T2D patients have shown a reduction in the cardiovascular risk profile. This can be achieved by lowering glucose, or it can be the result of moderate weight loss, or both. Metformin alone is known not to regenerate pancreas or liver in patients with T2D, nor does it directly affect the cardiovascular system or vascular endothelium. When we examined our control patients treated with metformin, we confirmed that even at a dose of 2.0 grams per day, there were no significant changes in any parameters indicative of regeneration. Specifically, the FS index increased with metformin alone and it slowly lost control of T2D in all parameters. see Figure 4 , Figure 5 , Figure 18 and Figure 19 , to account for the rise in the FS index and the loss of ...
Embodiment 3
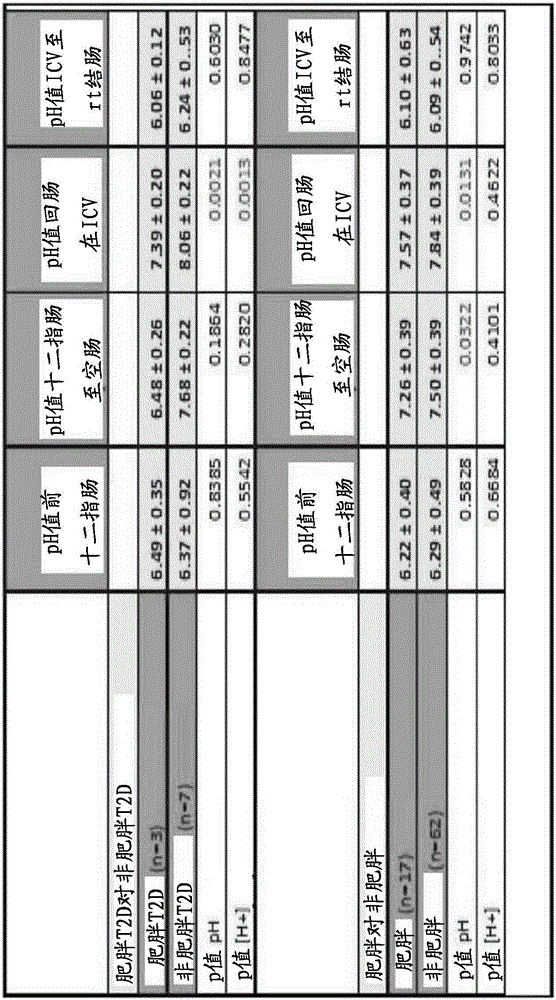
[0496] Example 3. Obesity and connection with gut microbiota
[0497] Use of the disclosed treatments and methods of modifying the human gastrointestinal flora for the purpose of triggering regeneration of pancreatic beta cells, liver cells and regeneration of gastrointestinal cells to facilitate treatment of metabolic syndrome based on the discovery. The probiotics selected for the coating in the formulation of the second active ingredient were Faecalibacterium prausnitzii, Bacteroides thetaiotaomicron, and Lactobacillus johnsonii. The approximate dose of these strains released in the ileum according to the formulation was 10^6 to 10^8 colony forming units. It is expected that these specific organisms will be co-formulated with typical probiotic bacterial organisms such as lactobacilli and bifidobacteria.
[0498] including Brake TM Clinical evidence of the utility of these synergistic combinations of diabetes therapies with probiotic replacement organisms will be provided...
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com