Conversion preparation method of euphorbia lathyris diterpene compound
A technology of capers and compounds, applied in the field of biopharmaceuticals, can solve the problems that have not yet been reported on the microbial transformation of capers diterpenoids
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
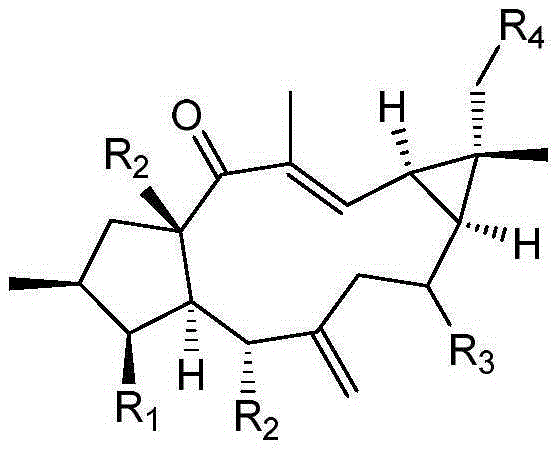
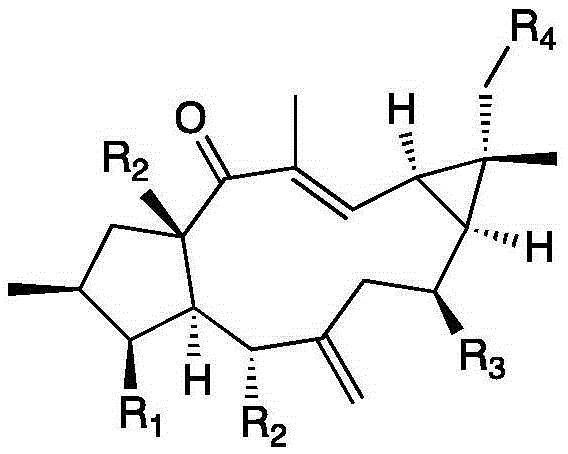
[0037] Example 1 Examination of the transformation results of Mucor circinelloides CICC 40242 on Lathyrol and Euphorbia Factor L3
[0038] The present invention transforms and screens 20 bacterial strains, and the result shows that the Mucor circinatus has strong transformation ability to the capricorn diterpene alcohol and euphorbia factor L3.
[0039] The strain screening medium is a potato medium: cut 200g of peeled potatoes into small cubes of 1 cubic centimeter, boil them with 1L of water for 20 minutes, add 20g of glucose after filtering the potato liquid, and distribute them in 250mL Erlenmeyer flasks, 50mL per bottle , sterilized at 121°C, 0.15Mpa for 20 minutes;
[0040] The strains were inoculated on the slant solid medium, cultured at 28°C for 7 days, and stored in a refrigerator at 4°C. The strains were activated by a two-step activation method. First, the strains were inoculated on the potato medium, and the flask was shaken at 28°C and 180rpm. After culturing fo...
Embodiment 2
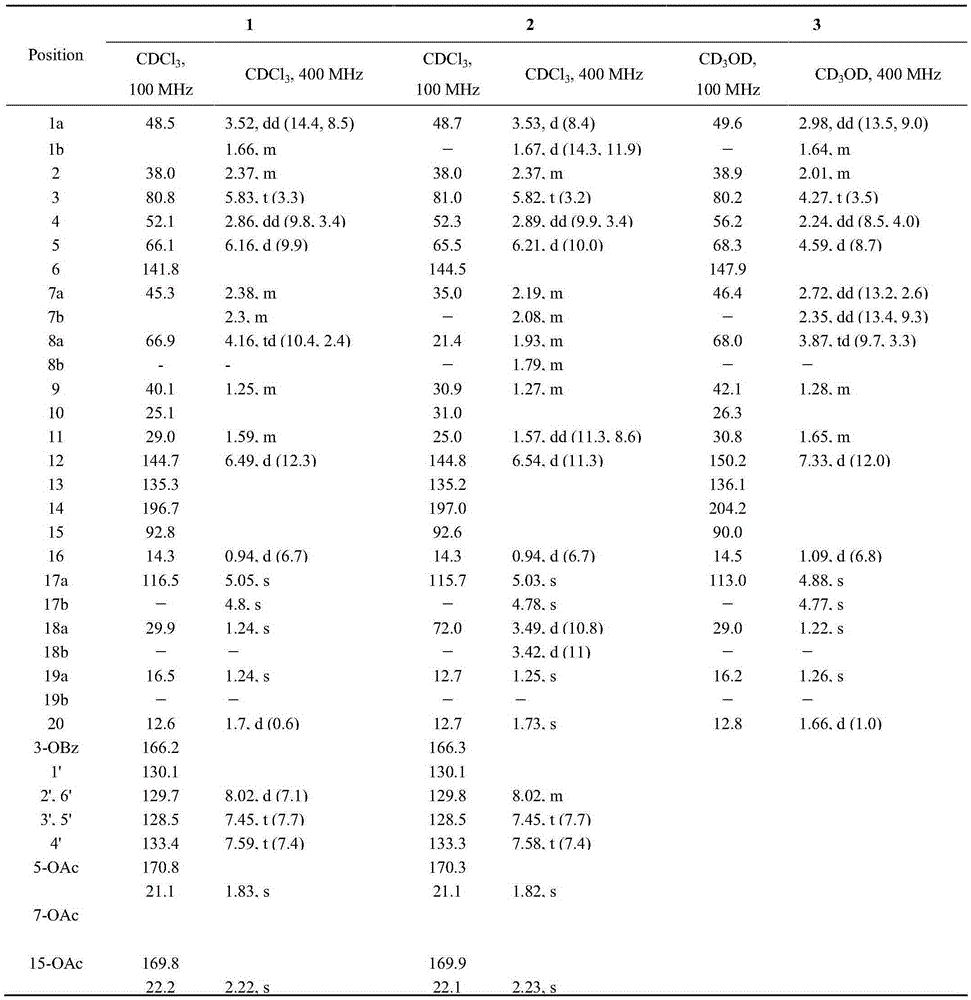
[0042] Example 2 Preparation of 8α-hydroxyeuphorbia factor L3(1) and 18-hydroxyeuphorbia factor L3(2)
[0043] The two-step activation method was used to activate the strains, and the obtained seed solution was inoculated into 250mL potato medium with a volume ratio of 1L in a 1L Erlenmeyer flask at a volume ratio of 1%, and was shaken at 28°C for 48h under the condition of 180rpm. Add 5 mg / mL Euphorbia factor L3 ethanol solution to the bacterial solution, and the final concentration is 0.1 mg / mL. After culturing under the same conditions for 72 hours, the fermentation broth was suction-filtered, the filtrate was extracted with ethyl acetate for 3 times, and the ethyl acetate was recovered after merging to obtain the total extract of the fermentation broth;
[0044] The total extract of the fermentation broth was dissolved with a small amount of ethyl acetate, mixed with 1g of silica gel (100-200 mesh), and loaded with dichloromethane wet method on a silica gel column equipped...
Embodiment 3
[0045] Example 3 Preparation of 8α-Hydroxy Capper Diterpene Alcohol (3)
[0046] The two-step activation method was used to activate the strains, and the obtained seed solution was inoculated into 250mL potato medium with a volume ratio of 1L in a 1L Erlenmeyer flask at a volume ratio of 1%, and was shaken at 28°C for 48h under the condition of 180rpm. Add 5 mg / mL ethanol solution of Caperia spp. diterpene alcohol to the bacterial liquid, the final concentration is 0.1 mg / mL, after 72 hours of cultivation under the same conditions, the fermentation liquid is suction-filtered, and the filtrate is extracted with ethyl acetate for 3 times, and the ethyl acetate is recovered after merging ester, to obtain the total extract of the fermentation broth;
[0047] The total extract of the fermentation broth is dissolved with a small amount of ethyl acetate, mixed with 1g of silica gel (100-200 mesh), the sample is loaded in dichloromethane wet method, and in a silica gel column equipped...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com