Application of lim kinase inhibitor limki3 in preparation of medicine for treating pain
A technology of kinase inhibitors and drugs, applied in the direction of drug combinations, antipyretics, anti-inflammatory agents, etc., can solve problems such as unmet needs for pain control, and achieve the effect of delaying pain phenotypes and treating pain
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
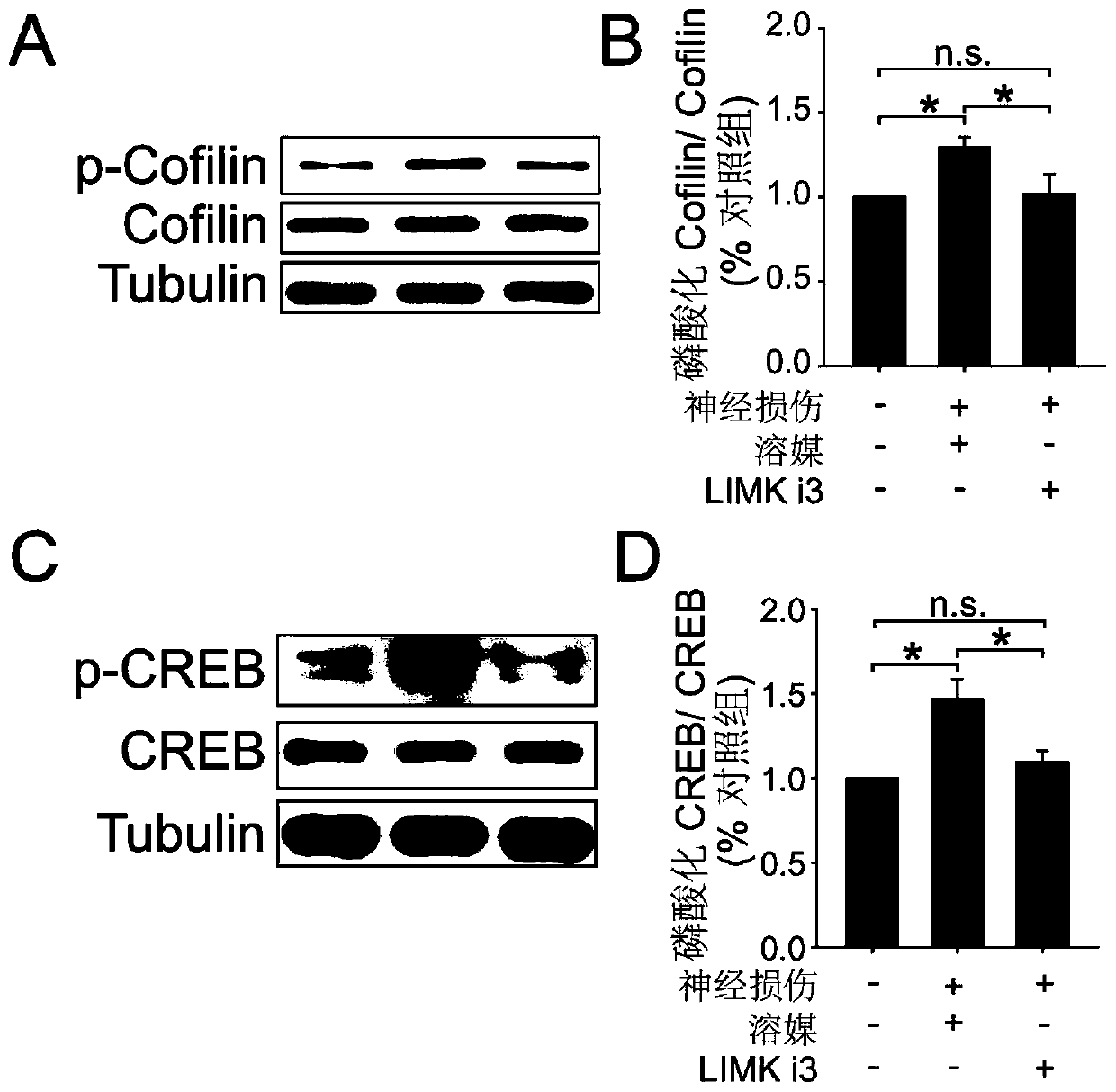
[0023] Example 1. Intrathecal administration of LIMKi3 can significantly inhibit the phosphorylation level of proteins downstream of LIM kinase
[0024] a) Test animals: healthy adult C57bl / 6 mice, clean grade, weighing 20-25 g. The experimental animals were raised in an independent environment with 12h-12h alternation of day and night, the room temperature was maintained at 24±2°C, water and food were free to drink, and the experiment was carried out after 1 week of adaptation to the environment. All handling of animals followed the requirements of the Ethics Committee of the International Association for the Study of Pain.
[0025] b) Experimental drug: the drug LIMKi3, with a purity >98%, is commercially available (purchased from Calbiochem). Dimethylsulfoxide (DMSO), commercially available. Tris, TEMED, Bovine Serum Albumin, β-Mercaptoethanol, glycine, glycerol, Tween-20, bromophenol blue, SDS, Triton 100, Marker were purchased from Sunshine; 30% Acrylamide / Bis, Ammonium...
Embodiment 2
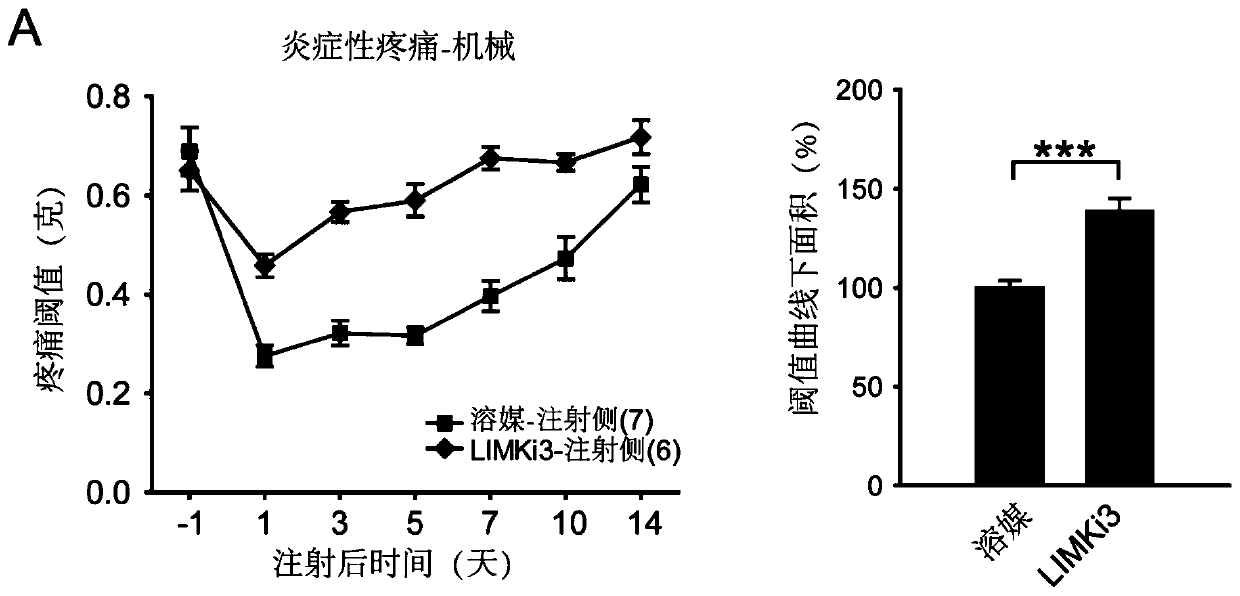
[0033] Example 2. Intrathecal administration of LIMKi3 can significantly inhibit the inflammatory pain phenotype
[0034] a) Test animals:
[0035] Healthy C57bl / 6 mice, clean grade, weighing 20-25g. The experimental animals were raised in an independent environment with 12h-12h alternation of day and night, the room temperature was maintained at 24±2°C, water and food were free to drink, and the experiment was carried out after 1 week of adaptation to the environment. All handling of animals followed the requirements of the Ethics Committee of the International Association for the Study of Pain.
[0036] b) Test drugs and reagents:
[0037] The drug LIMKi3, with a purity >98%, is commercially available (purchased from Calbiochem). Dimethylsulfoxide (DMSO), commercially available.
[0038] c) Test method:
[0039] Inflammatory pain model: After the mice were preserved, inject 20 microliters of a 1:1 mixture of complete Freund's adjuvant and saline into the soles of the mice...
Embodiment 3
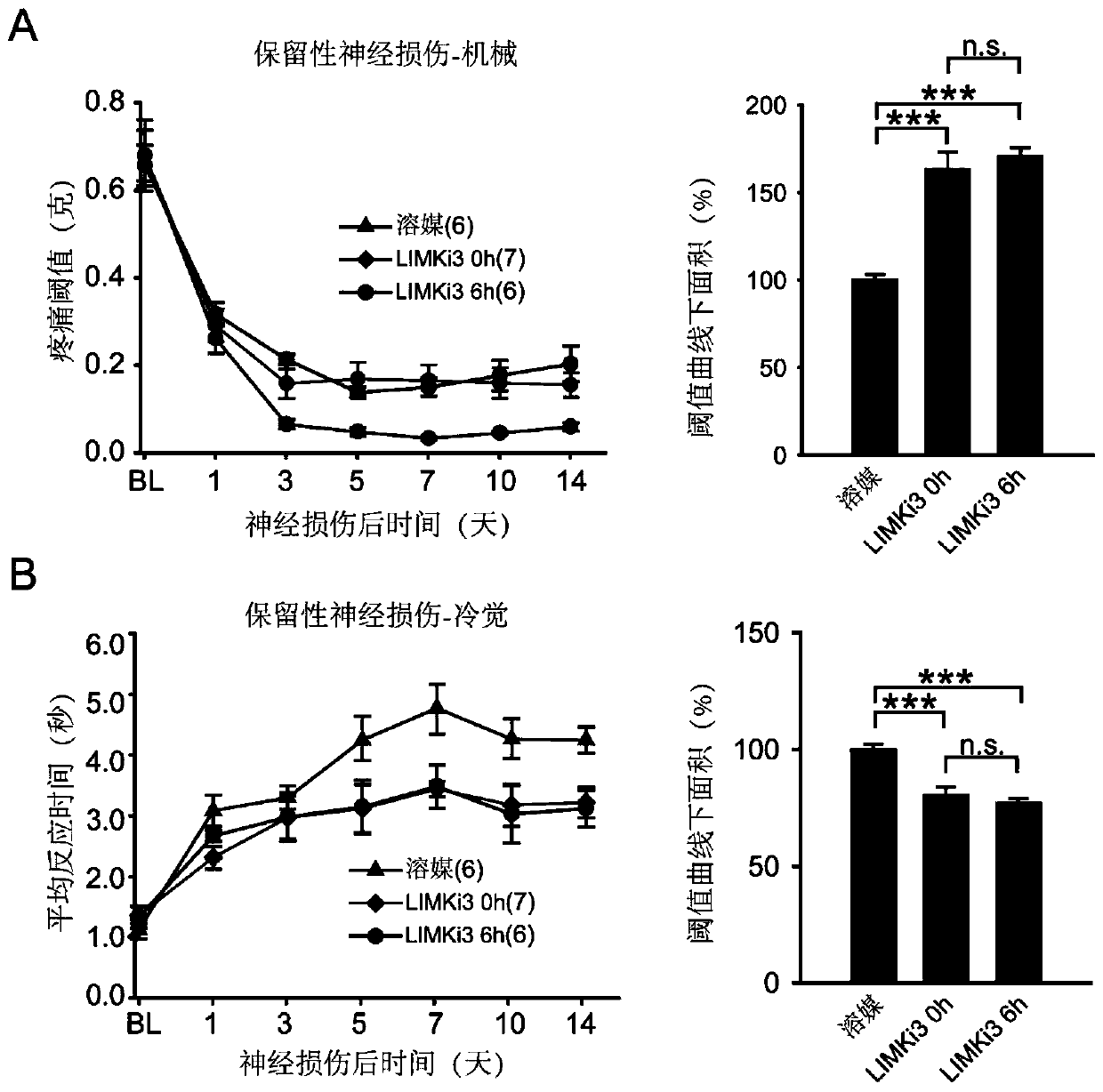
[0046] Example 3. The effect of intrathecal administration of LIMKi3 on the pain phenotype of preserved nerve injury
[0047] a) Test animals:
[0048] Healthy C57bl / 6 mice, clean grade, weighing 20-25g. The experimental animals were raised in an independent environment with 12h-12h alternation of day and night, the room temperature was maintained at 24±2°C, water and food were free to drink, and the experiment was carried out after 1 week of adaptation to the environment. All handling of animals followed the requirements of the Ethics Committee of the International Association for the Study of Pain.
[0049] b) Test drugs and reagents:
[0050] The drug LIMKi3, with a purity >98%, is commercially available (purchased from Calbiochem). Dimethylsulfoxide (DMSO), commercially available.
[0051] c) Test method:
[0052] Preserved nerve injury model: After mice are anesthetized, cut the skin and bluntly separate the muscles to see the main trunk of the sciatic nerve and its ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com