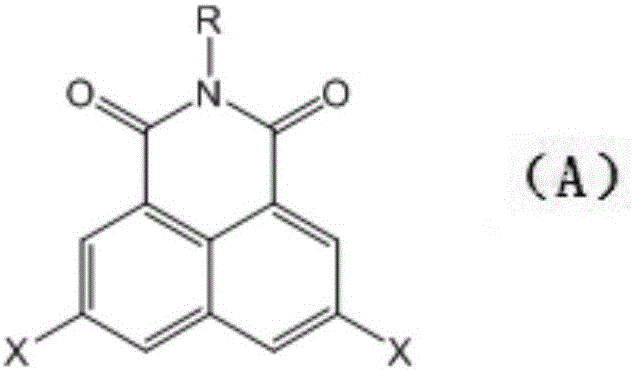
Synthesis method of 3,6-halogen atom-substituted 1,8-naphthalimide
A technology of naphthalimide and synthesis method, applied in the direction of organic chemistry, etc., can solve the problems of difficult separation and purification, harsh preparation conditions, etc., and achieve the effect of simple separation and purification and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
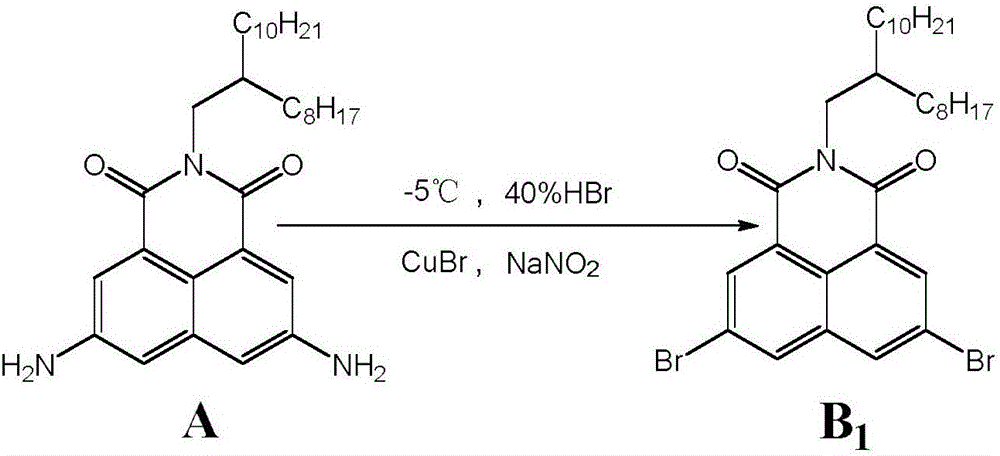
[0016] Example 1: 3,6-dibromo-N-(2-octyldodecyl)-1,8-naphthalimide (B 1 )
[0017]
[0018] In a 50mL flask, add reactant A (0.102g, 0.20mmol), 7mL 42% hydrobromic acid aqueous solution, stir it with a magnetic stirrer to fully form a salt, and then slowly add 0.110g dissolved in 4mL water in an ice-salt bath (1.59mmol)NaNO 2 After dripping, stir under ice-salt bath for 0.5h, then slowly add 0.110g (0.77mmol) CuBr solution dissolved in 6mL of 42% hydrobromic acid aqueous solution, stir and react until the solution is clear or stir overnight, react After stopping, it was extracted with dichloromethane, washed with water, dried with anhydrous magnesium sulfate, filtered, and separated by column to obtain 0.058 g of light yellow solid with a yield of 45.4%. 1 HNMR (300MHz, CDCl 3 )δ H :8.63(s,2H),8.26(s,2H),4.07(d,2H),1.22-1.29(m,33H),0.85-0.87(m,6H); HRMS: Calcd for C 32 h 45 Br 2 NO 2 633.1817, found: 633.1836 (M - , MALDI-TOF).
Embodiment 2
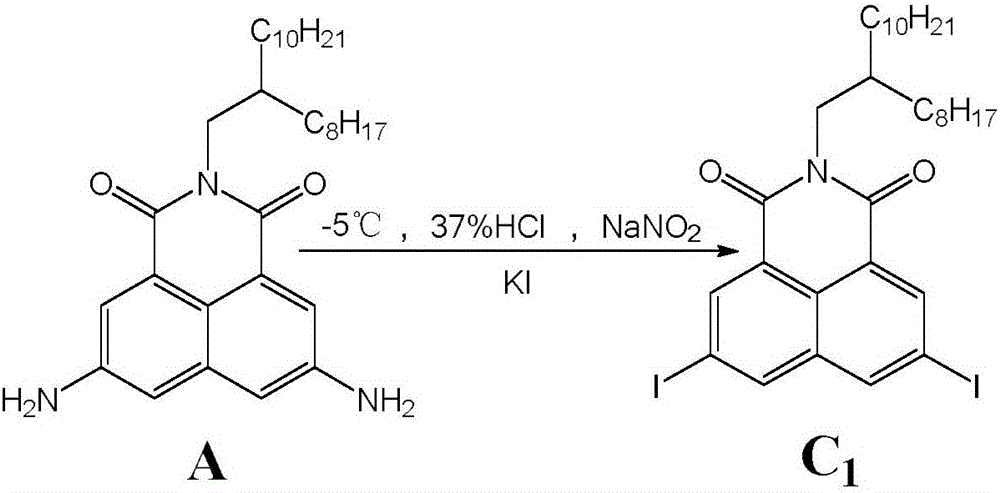
[0019] Example 2: 3,6-diiodo-N-(2-octyldodecyl)-1,8-naphthalimide (C 1 )
[0020]
[0021] In a 50mL flask, add reactant A (0.102g, 0.20mmol), 7mL of 37% hydrochloric acid aqueous solution, stir it with a magnetic stirrer to make it fully salt, then slowly add dropwise 0.110g (1.59 mmol)NaNO 2 After dripping, stir in an ice-salt bath for 0.5h, then slowly add 0.30g (1.81mmol) KI solution dissolved in 5mL of water dropwise, stir until the solution is clear or stir overnight, and extract with dichloromethane after the reaction stops , washed with water, dried with anhydrous magnesium sulfate, filtered, and column separated to obtain 0.060 g of a dark red solid with a yield of 41.2%. 1 HNMR (300MHz, CDCl 3 )δ H :8.78(s,2H),8.45(s,2H),4.07(d,2H),1.22-1.29(m,33H),0.85-0.87(m,6H); HRMS: Calcd forC 32 h 45 I 2 NO 2 729.1540, found: 729.1522 (M - , MALDI-TOF).
Embodiment 3
[0022] Example 3: 3,6-dibromo-N-(2-octyldodecyl)-1,8-naphthalimide (B 1 )
[0023]
[0024]In a 50mL flask, add reactant A (0.102g, 0.2mmol), 7mL 42% hydrobromic acid aqueous solution, add 7mL water and stir it with a magnetic stirrer to fully form a salt, then add 7mL THF, and then Slowly add 0.110g (1.59mmol) NaNO dissolved in 4mL of water dropwise 2 After dripping, stir under ice-salt bath for 0.5h, then slowly add 0.110g (0.77mmol) CuBr solution dissolved in 6mL of 42% hydrobromic acid aqueous solution, stir and react until the solution is clear or stir overnight, react After stopping, it was extracted with dichloromethane, washed with water, dried with anhydrous magnesium sulfate, filtered, and separated by column to obtain 0.051 g of a light yellow solid with a yield of 40%. 1 HNMR (300MHz, CDCl 3 )δ H :8.63(s,2H),8.26(s,2H),4.07(d,2H),1.22-1.29(m,33H),0.85-0.87(m,6H); HRMS: Calcd for C 32 h 45 Br 2 NO 2 633.1817, found: 633.1836 (M - , MALDI-TOF).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com