Synthesis method of N-aryl hydroxylamine acid
A technology for the synthesis of aryl hydroxyamic acid and its method, which is applied in the field of synthesis of N-aryl hydroxyamic acid, can solve the problems of harsh operating conditions, difficult handling, and moisture sensitivity of acyl chlorides, and achieve mild operating conditions and simple separation and purification operations Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033]
[0034] The specific process is as follows:
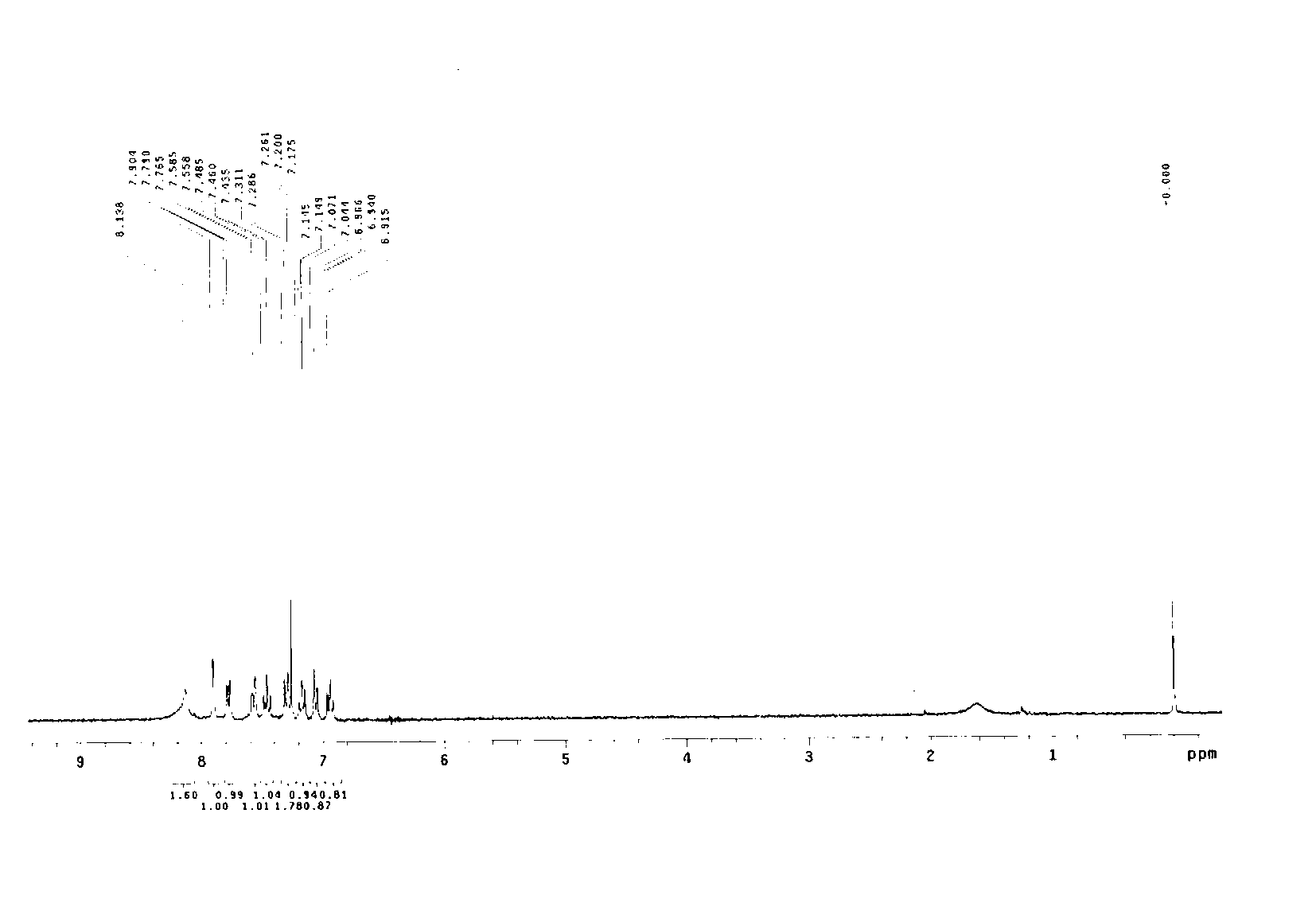
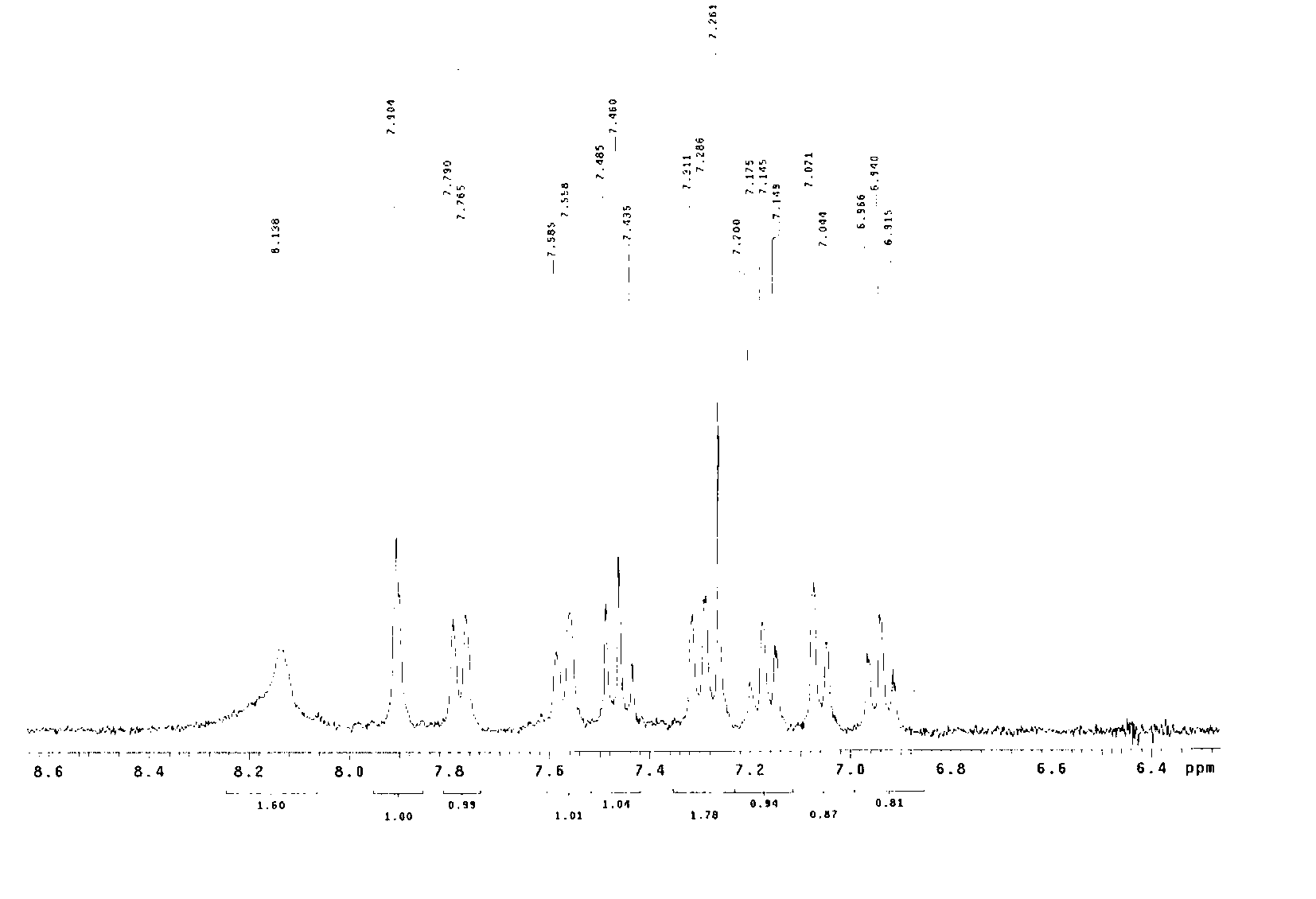
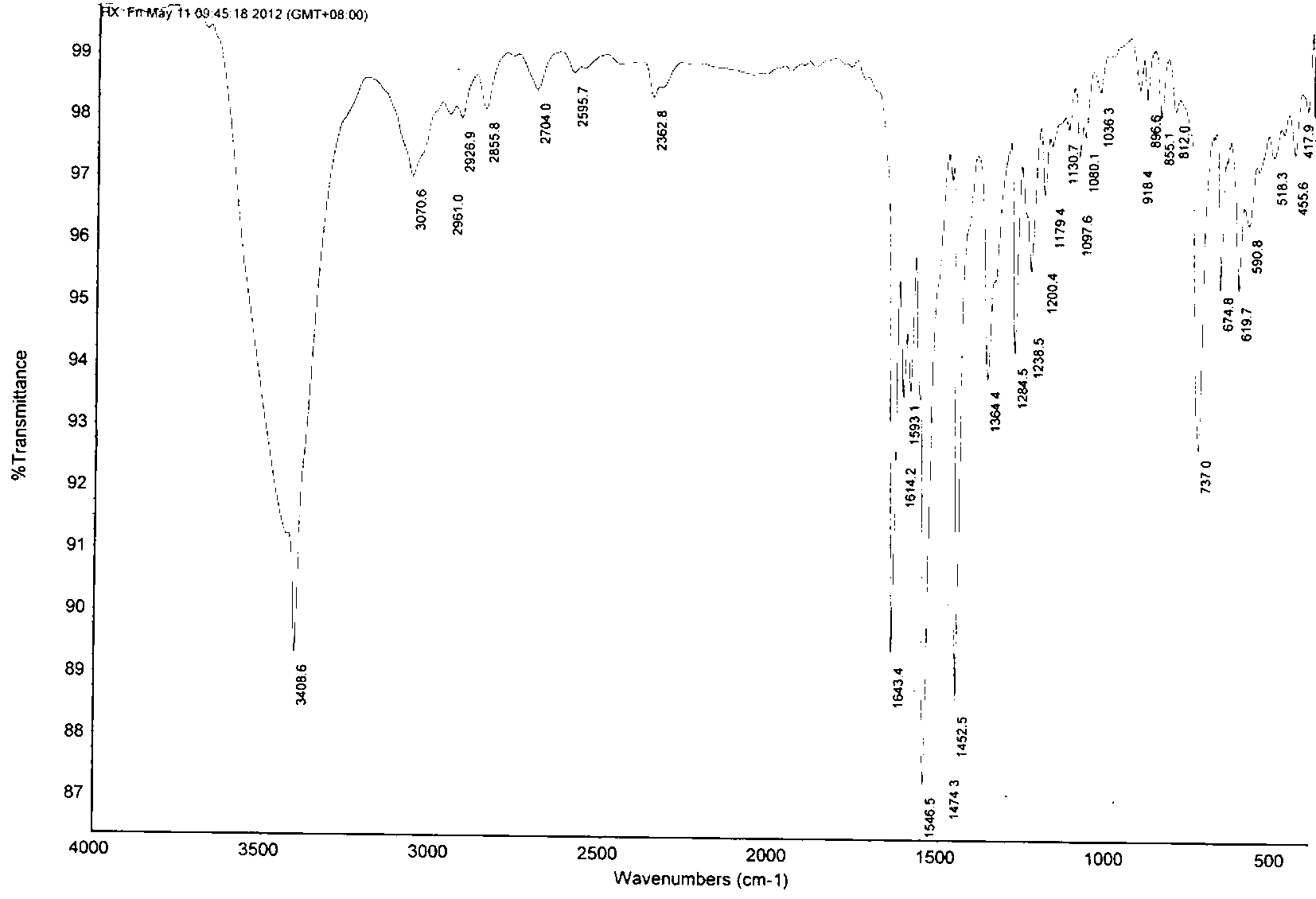
[0035] In a 100mL single-necked flask, add 50mL of dried dichloromethane, and then add 0.465g of aniline and 0.530g of benzaldehyde in sequence, and the molar ratio of aniline and benzaldehyde is 1:1. Stir well at room temperature, react for 6 hours, add 50 mL of saturated saline, and quench the reaction. It was extracted three times with dichloromethane, and 50 mL of dichloromethane was used each time. The organic phases were combined, dried with anhydrous sodium sulfate, and evaporated to dryness of dichloromethane to obtain a solid, namely intermediate A, with a yield of 95%.
[0036] Take 197 mg of intermediate A, add it to 10 mL of dried dichloromethane, stir to dissolve, then place it in an ice-water bath (0°C), add 102 mg of sodium bicarbonate and 258 mg of m-chloroperoxybenzoic acid (m- CPBA), after stirring for half an hour, the temperature of the reaction solution rose to room temperature, and after 12 hours ...
Embodiment 2
[0038]
[0039] The specific process is as follows:
[0040] In a 100mL single-necked flask, add 50mL of dried dichloromethane, and then add 0.465g of aniline and 0.660g of cinnamaldehyde in sequence, and the molar ratio of aniline and cinnamaldehyde is 1:1. Stir well at room temperature, react for 6 hours, add 50 mL of saturated saline, and quench the reaction. It was extracted three times with dichloromethane, and 50 mL of dichloromethane was used each time. The organic phases were combined, dried with anhydrous sodium sulfate, and evaporated to dryness of dichloromethane to obtain a solid, namely intermediate B, with a yield of 98%.
[0041] Take 207 mg of intermediate B, add it to 10 mL of dried dichloromethane, stir to dissolve, then place it in an ice-water bath (0°C), add 102 mg of sodium bicarbonate and 258 mg of m-chloroperoxybenzoic acid (m- CPBA), after stirring for half an hour, the temperature of the reaction solution rose to room temperature, and after 12 ho...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com