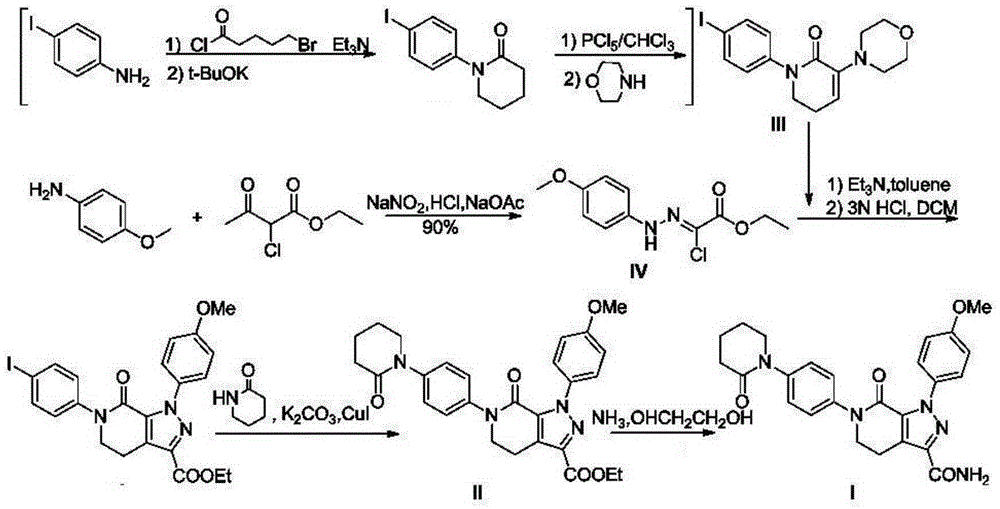
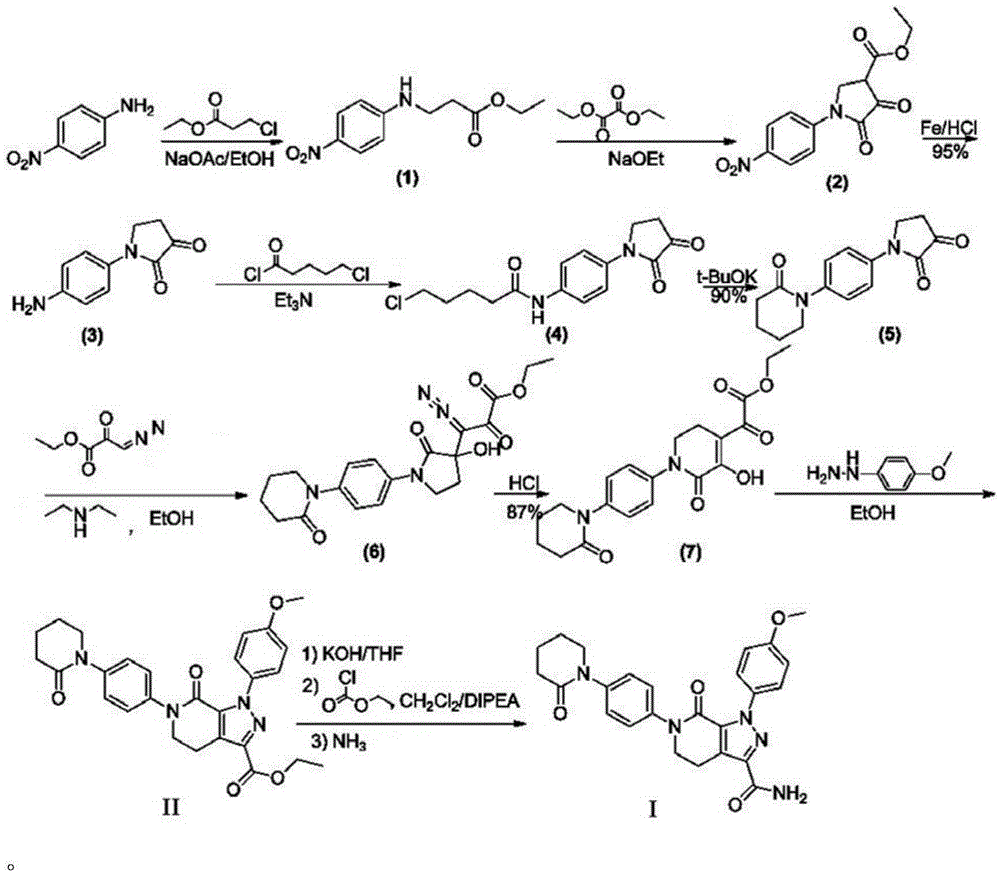
A kind of synthetic method of Apixaban
A synthetic method and technology of apixaban, applied in the field of drug synthesis, can solve the problems of only reaching 29%, high price, unfavorable cost, etc., and achieve the effects of simplifying separation and purification operations, reducing synthesis costs, and short reaction time.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Compound 1: Synthesis
[0046] Add 138g (1mol) of p-nitroaniline, 700ml of ethanol, 163g (1.2mol) of ethyl 3-chloropropionate to the reaction bottle, add 82g (1mol) of sodium acetate in small amounts at 40°C, and keep the temperature for reaction For 3 to 5 hours, LCMS followed the reaction until the reaction of p-nitroaniline was complete. The reaction liquid was cooled to 0-5oC in an ice-water bath, and 2L of water was added to stir and crystallize, filtered with suction, and vacuum-dried at 40°C for 4 hours to obtain 214g of compound 1, mol Yield: 90%.
[0047] Compound 2: Synthesis
[0048] Add 119g (0.5mol) of compound 1, 600ml of ethanol, 80.3g (0.55mol) of diethyl oxalate to the reaction flask, add 6.8g (0.1mol) of sodium ethoxide in small amounts at 52°C, and keep the temperature for reaction 2 -3 hours, LCMS followed the reaction until the reaction of compound 1 was complete, the reaction solution was cooled to 0-5°C in an ice-water bath, neutralized with...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com