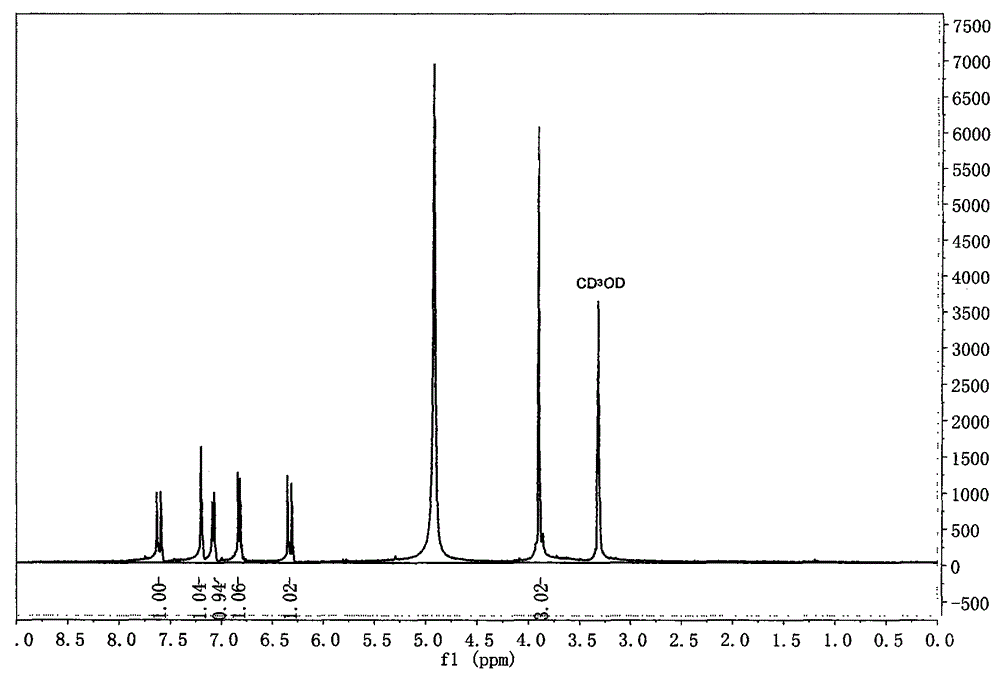
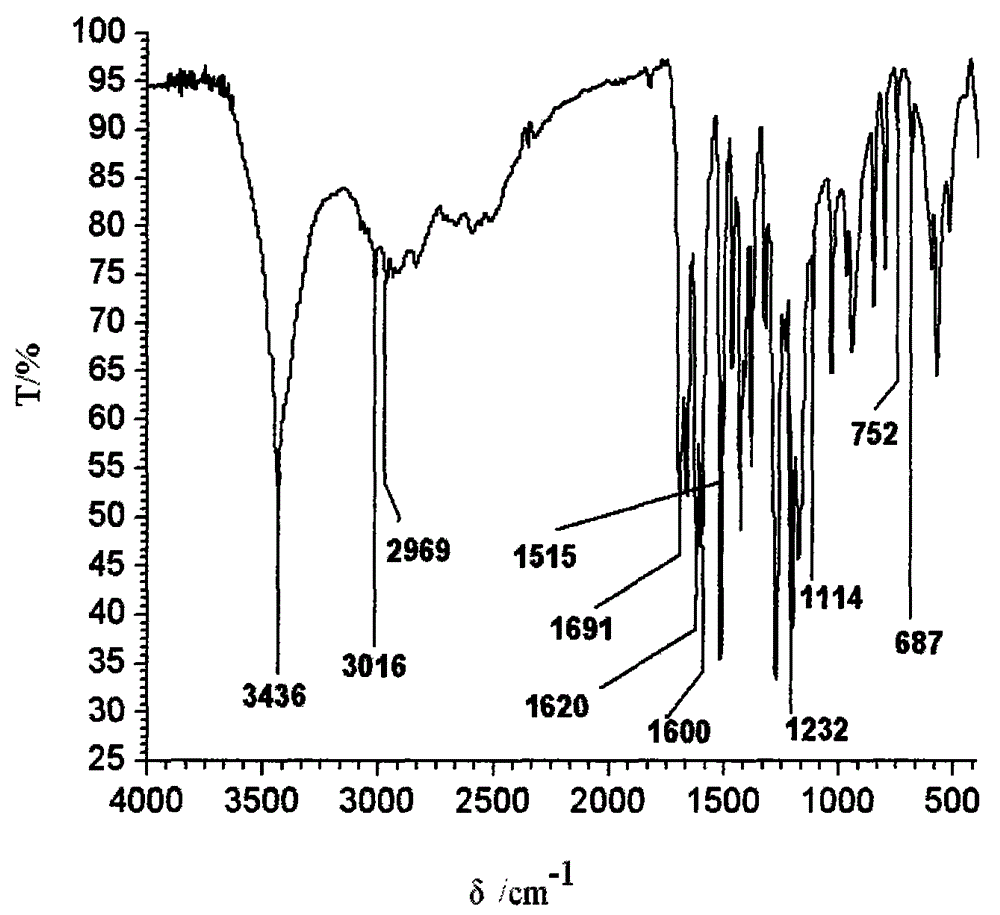
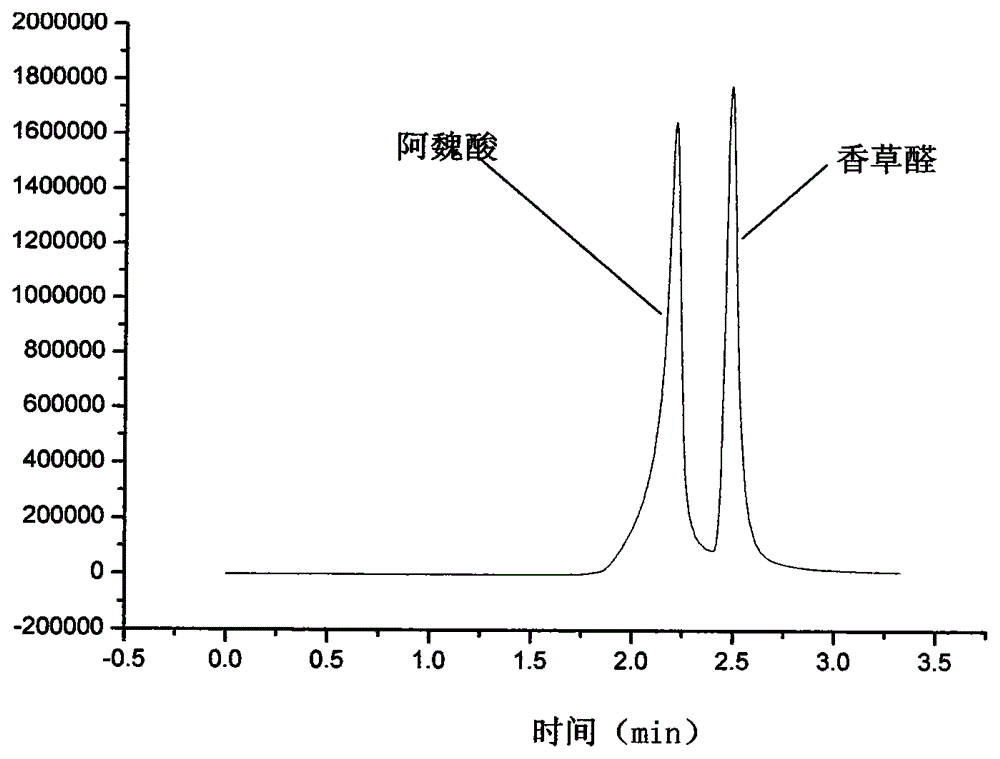
Method for synthesizing ferulic acid by microwave radiation
A technology of microwave radiation and ferulic acid, which is applied in the field of synthesis of ferulic acid, can solve the problems of long reaction time and achieve the effects of short reaction time, expansion of the reaction system, and simple separation and purification operations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Embodiment 1 The method for the synthesis of ferulic acid by microwave radiation
[0035] Put 20mmol vanillin, a certain amount of acetic anhydride (that is, reactant is also solvent) and 20mmol Anhydrous Potassium Carbonate in dry round-bottomed flask, after shaking up, put into microwave chemical reactor, install reflux condenser, Under anhydrous conditions, under a certain microwave power, microwave radiation was continued for several minutes until the reaction mixture was completely melted into a liquid. The molten liquid was cooled to room temperature to obtain a solidified product, and about 70 mL of ice water was added to the obtained solid, shaken and mixed, left to stand for one day, fully washed with about 200 mL of ice water and suction filtered. The filter cake was hydrolyzed in 20% NaOH solution at 50°C for 20 minutes, and filtered with suction. The obtained filtrate was acidified with hydrochloric acid to pH = 2-3, filtered with suction to obtain a filter...
Embodiment 2
[0045] Embodiment 2 The influence of acetic anhydride consumption on productive rate
[0046] Carry out the method for synthesizing ferulic acid according to embodiment 1, vanillin consumption is 3.0g (20mmol), anhydrous potassium carbonate 2.8g (20mmol), microwave power 240W, microwave time 10min, investigate the influence of acetic anhydride consumption on productive rate , see the result Figure 4 . Depend on Figure 4 It can be seen that the productive rate increases with the increase of the amount of acetic anhydride. When the amount of acetic anhydride is 200mmol, the productive rate is the largest, and when it exceeds 200mmol, the productive rate decreases instead. This may be because the amount of acetic anhydride used is too much, which reduces the catalytic effect of the catalyst. The amount of acetic anhydride is too small, for example, when the acetic anhydride is 60mmol, the liquid after the reaction is black. This may be because there is no other solvent in th...
Embodiment 3
[0047] Example 3 Effect of Microwave Power on Productivity
[0048] Carry out the method for synthesizing ferulic acid according to embodiment 1, vanillin consumption is 3.0g (20mmol), anhydrous potassium carbonate 2.8g (20mmol), acetic anhydride 200mmol, microwave time 10min, investigate the influence of microwave power on productive rate, see results Figure 5 . Depend on Figure 5 It can be seen that with the increase of the radiation power, the yield increases, and the increase of the microwave power promotes the increase of the effective collision between the reactants, which is beneficial to the increase of the yield. Depend on Figure 5 It can also be seen that when the radiation power exceeds 400W, the yield increases slowly. From the consideration of saving energy, the microwave radiation power is more suitable for 400W.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com