Human NOTCH1 NICD protein antigen and antibody as well as preparation methods and application thereof
A protein and antibody technology, applied in the direction of animal/human protein, anti-animal/human immunoglobulin, antibody, etc., can solve the problems of abnormal phosphorylation modification, half-life extension, etc., and achieve high affinity, good recovery rate, and short preparation cycle short effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
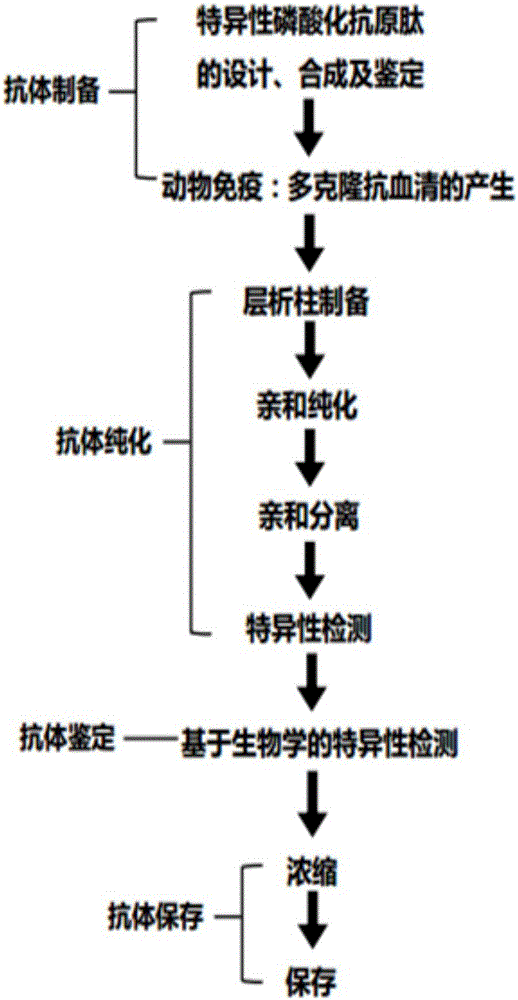
[0041] see figure 1 , the preparation method and application of the phosphorylated antibody against human NOTCH1 NICD protein Thr1861 site provided by the embodiment of the present invention generally includes the following steps:
[0042] Step 1: Antigen epitope design and screening: The inventor selected the SEQ ID of the intracellular segment protein (NICD) in the human NOTCH1 protein through long-term accumulated experience, which has the ability to induce animals to produce highly antigenic and highly hydrophilic antibodies NO: 1 polypeptide, and the threonine (Thr) at position 1861 was phosphorylated and modified.
[0043] Step 2: Preparation of synthetic peptides and coupled proteins: Synthesize the antigenic peptide shown in SEQ ID NO: 1 using peptide synthesis technology, add a phosphorylated group to the amino acid Thr1861, and a cysteine is connected to the C-terminus (obtained said antigen peptide), after HPLC purification and mass spectrometry (MS) identificati...
Embodiment 1
[0048] Example 1 Antigen epitope design and screening
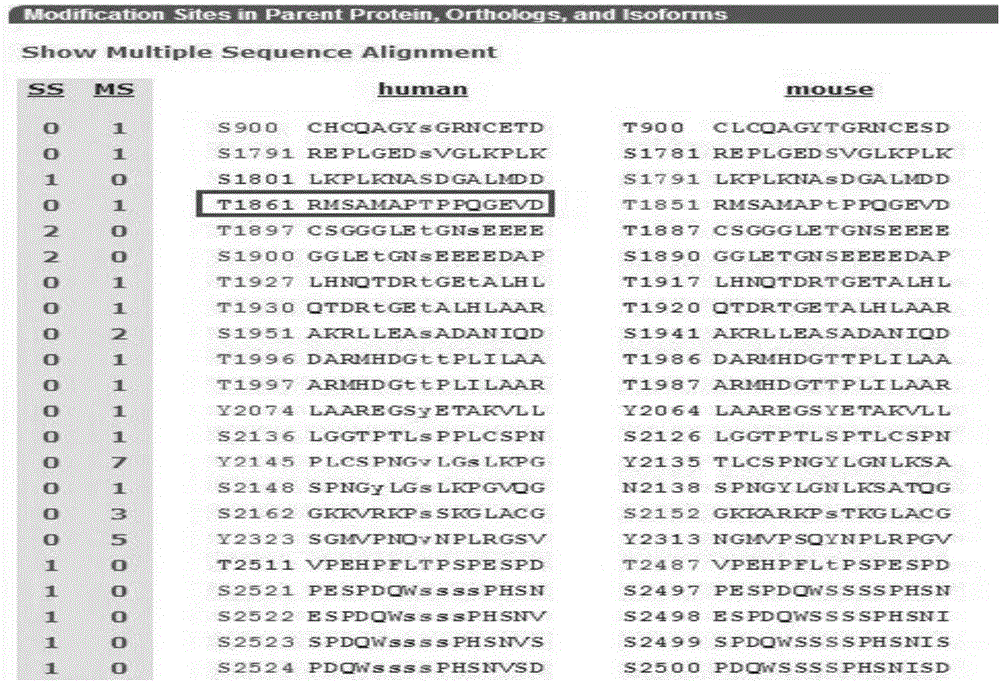
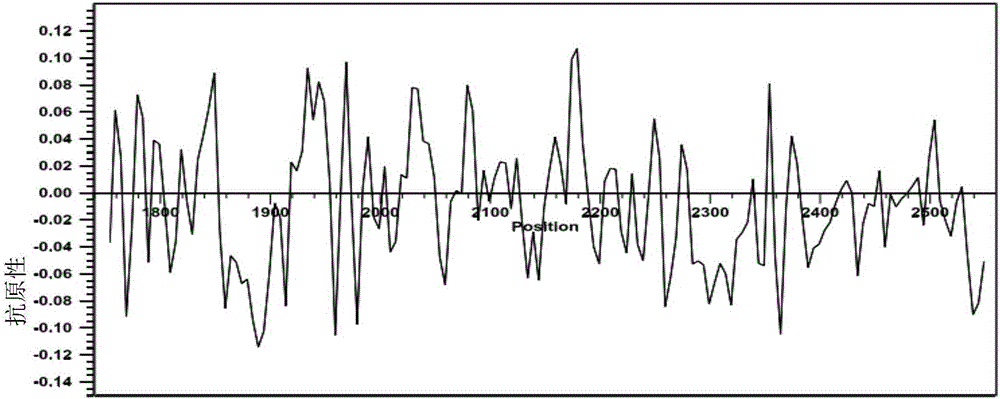
[0049] 1.1 The inventors of the present invention have screened for the ability of highly specific and highly hydrophilic antibodies phosphorylated at the Thr1861 site of the human NOTCH1 NICD protein. This epitope contains 9 amino acids (aa), and the sequence is as shown in SEQ ID NO: Shown in 1: N-Ala Met Ala Pro p(Thr)Pro Pro Gln Gly-C; Finally, the homology analysis of the synthetic peptide was carried out using blastp on the NCBI website, and the results showed that the peptide sequence of the antigenic epitope was no different from that of rabbits origin, and calculate the antigenicity and hydrophilicity of the human NOTCH1 amino acid sequence ( image 3 , Figure 4 ).
Embodiment 2
[0050] Embodiment 2 Synthetic peptide and coupling protein preparation
[0051] According to the design of the epitope peptide in Example 1, a phosphorylation group was added to the Thr1861 site and a cysteine was added to the C-terminus of the sequence to obtain a phosphorylated synthetic peptide (SEQ ID NO: 2), which was analyzed by HPLC. After purification, a synthetic peptide with a purity of 96% was obtained, which was identified by mass spectrometry and was consistent with the design ( Figure 5 ), and then through the C-terminal cysteine and hemocyanin (KLH) coupling to obtain the whole antigen (pT1861-KLH, Thr referred to as T) will be used for animal immunization; at the same time, the synthetic peptide through the C-terminal cysteine and After coupling with bovine serum albumin (BSA), it was used for the isolation of phosphorylated antibody (pT1861-BSA). Similarly, a synthetic peptide as shown in SEQ ID NO: 1 is synthesized by using peptide synthesis technolog...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com