Internal standard detection method for furacilin biomarker NF (5-nitro-2-furaldehyde)
A nitrofurazone biological and detection method technology, applied in the detection field of nitrofurazone biomarker 5-nitro-2-furfural, can solve problems such as indistinguishability, and achieve the effect of breaking through limitations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
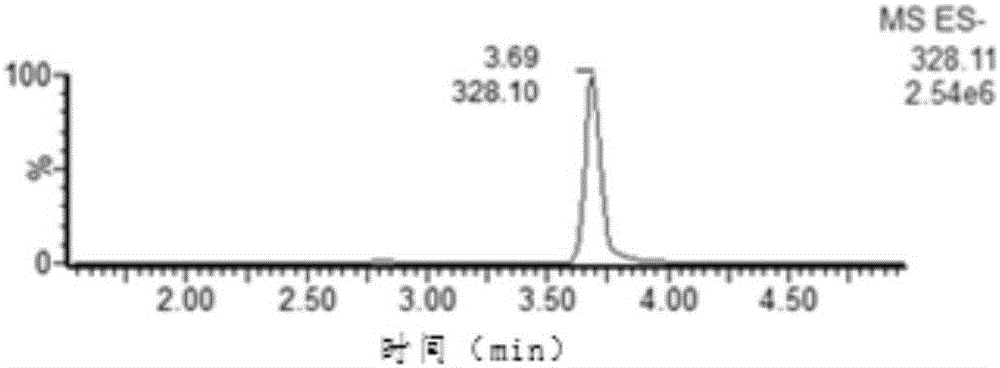
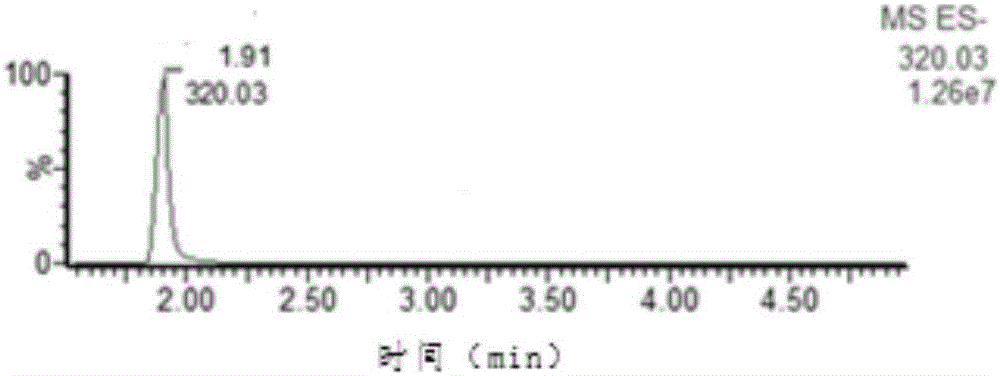
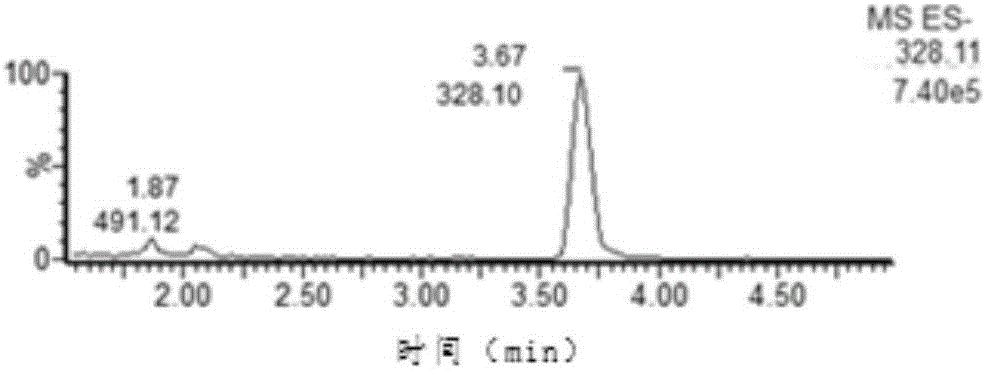
[0028] An internal standard detection method of nitrofurazone biomarker 5-nitro-2-furfural, comprising the steps of:
[0029] (1) Reagent preparation
[0030] Dissolve 100 mg of DNPH (2,4-dinitrophenylhydrazine) in 1 ml of concentrated hydrochloric acid (12M), then dilute to a 100 ml volumetric flask with methanol to obtain a derivatization concentration of 1.0 mg / mL Reagent solution;
[0031] (2) Derivatization and purification
[0032] Weigh 1.0±0.1g of sample, put it into a 50ml sample tube, add 10ml of hydrochloric acid (0.2mol / L), 100μL of derivatization reagent solution, 100μL of PDAB solution (1.0μg / mL), vortex fully, avoid Light was placed in a water bath at 60°C for 20 minutes, followed by centrifugation at 6000 rpm for five minutes, and the supernatant was purified by Oasis PRiME HLB solid-phase extraction cartridge (Waters), and the obtained liquid was rotary evaporated to dryness. The residue was redissolved with 1.0 ml of mobile phase (acetonitrile / water=7 / 3, V...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com