Method of quantifying content of recombinant troponin I by peptide fragment isotope dilution mass-spectrography
A technology of isotope dilution and troponin, which is applied in the field of peptide isotope dilution mass spectrometry to quantify the content of recombinant troponin I, can solve the problems of the influence of ionization degree, complex experimental conditions, and potential safety hazards
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0036] In order to make the objectives, technical solutions and advantages of the present invention clearer, the present invention will be further described in detail below in conjunction with specific embodiments. It should be understood that the specific embodiments described herein are only used to explain the present invention, and not to limit the present invention.
[0037] This specific embodiment adopts the following technical scheme: a method for quantifying the content of recombinant troponin I by peptide isotope dilution mass spectrometry, including the following steps:
[0038] (1) Peptide design:
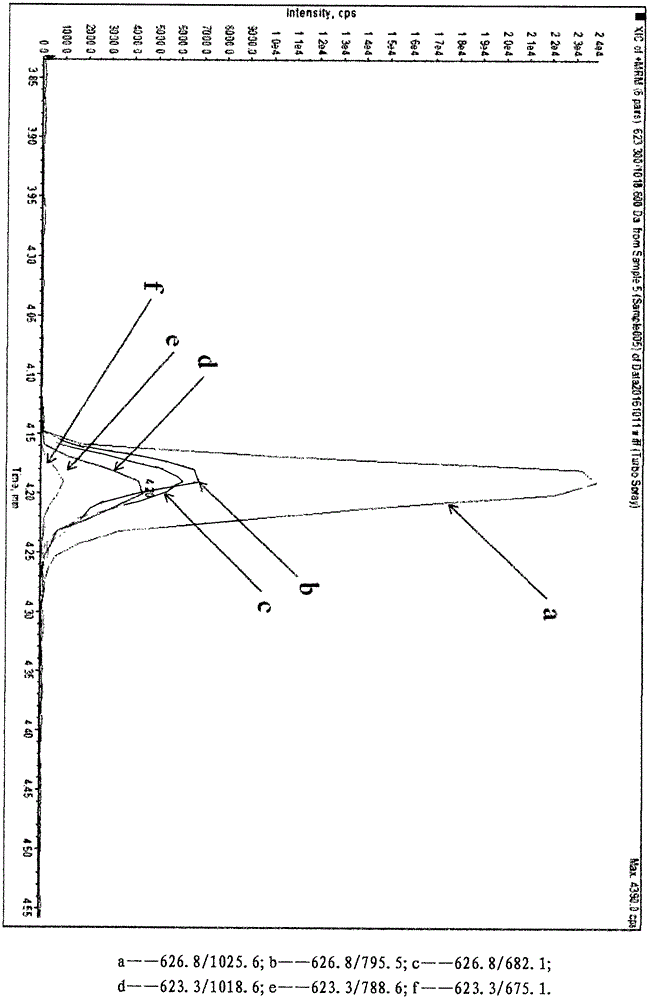
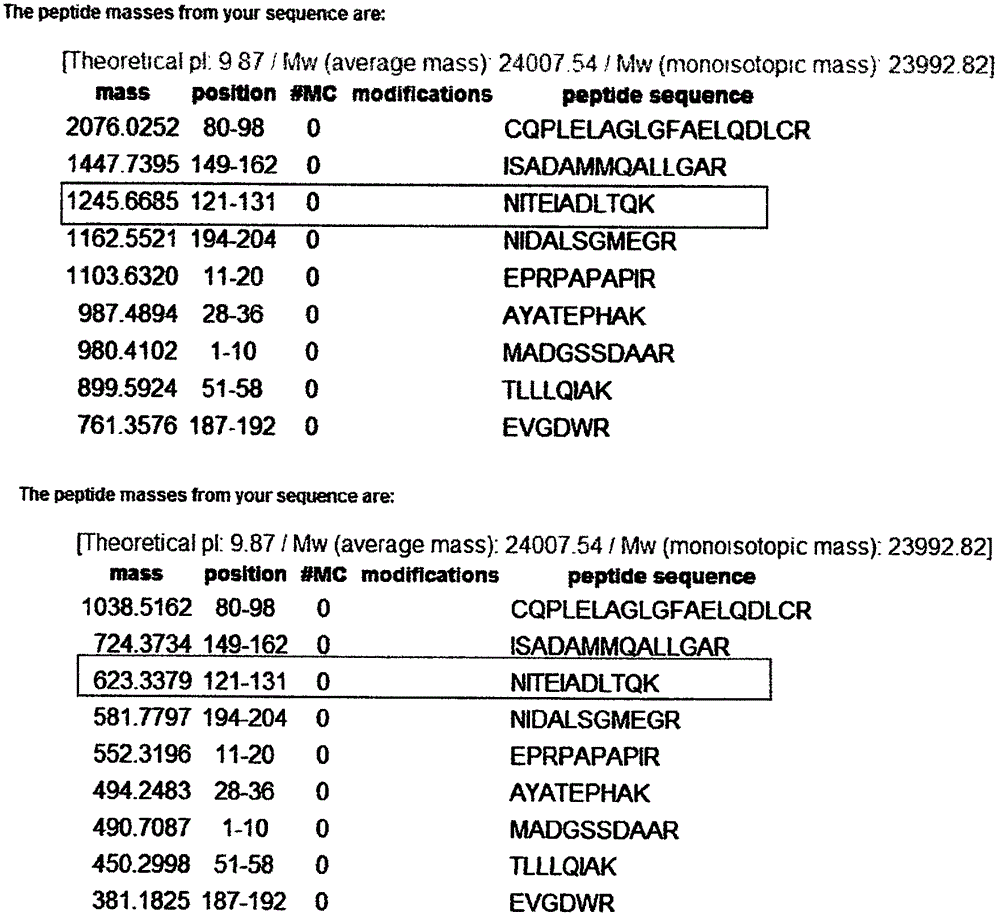
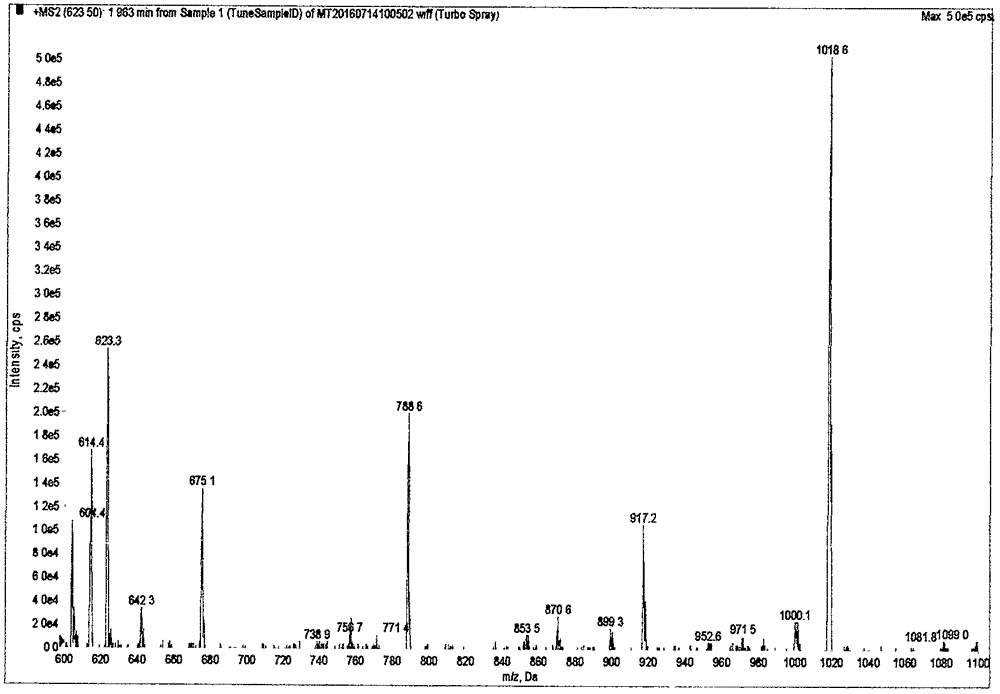
[0039] According to the amino acid sequence of human cTnI protein, use Peptide mass software to design isotope dilution mass spectrometry to detect cTnI specific peptide NITEIADLTQK, see figure 1 , The peptide was searched by BLAST as a specific peptide with a theoretical molecular weight of 1245.7 Da. The synthetic peptide has a molecular weight of 1245.41 Da, which is main...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com