Amino functional group chiral compound resolution labeling with fluorescent derivatization reagent
A chiral compound, fluorescence derivatization technology, applied in the field of biological analysis, can solve the problem of not achieving complete chiral resolution, and achieve the effect of reliable fluorescent chiral derivatization reagents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
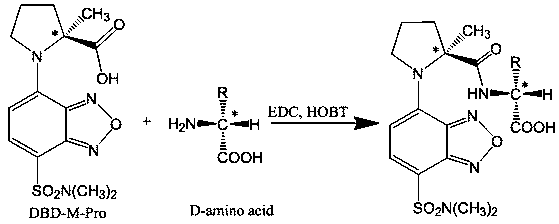
[0018] Synthesis of DBD-M-Pro:
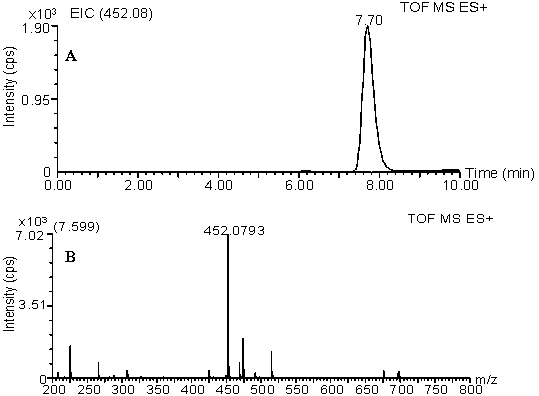
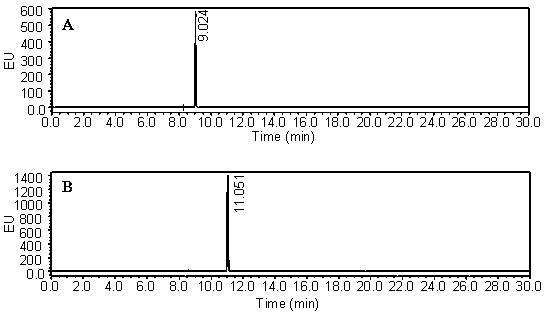
[0019] Dissolve DBD-F 100 mg in 10 mL of acetonitrile solution, M-L-Pro 54 mg in 10 mL of 0.25 M Na 2 CO 3 The two solutions were mixed, reacted at 30° C. for 1 hour on a constant temperature magnetic stirrer, and the solvent was distilled off under reduced pressure. The resulting residue was re-dissolved in 20 mL of water, and then the same volume of ethyl acetate was added. After sufficient static, the ethyl acetate layer was discarded, and the pH of the aqueous layer was adjusted to 1-2 with 2.0 mol / L HCl. Then, an equal amount of ethyl acetate was added for extraction, and the extraction was repeated three times. The combined ethyl acetate layers were dried over anhydrous sodium sulfate, filtered, and evaporated to dryness under reduced pressure to obtain 78.6 mg of yellow powder. LC-ESI-TOF-MS spectral data, (m / z): 355.06 [M+H] + , t R =6.23 min; 1 H-NMR (300 MHz, CDCl 3 ) δ 7.89 (t, J = 14.2 Hz, 1H), 6.00 (d, J = 8.2 Hz, 1H), 4...
PUM
| Property | Measurement | Unit |
|---|---|---|
| separation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com