Time-resolved fluoroimmunoassy test strip used for quantitative determination of soluble growth stimulation gene-2 (sST2), and preparation method thereof
A time-resolved fluorescence and quantitative detection technology, applied in the field of medical testing, can solve the problems that the soluble growth-stimulating gene 2 protein is not suitable for clinical rapid diagnosis, high requirements for testing equipment, and many interference factors, so as to facilitate the calculation of sample concentration and the detection time. The effect of short, accurate regression equations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] The preparation method of the time-resolved fluorescent immunoassay test strip for quantitatively detecting sST2 comprises the following steps:
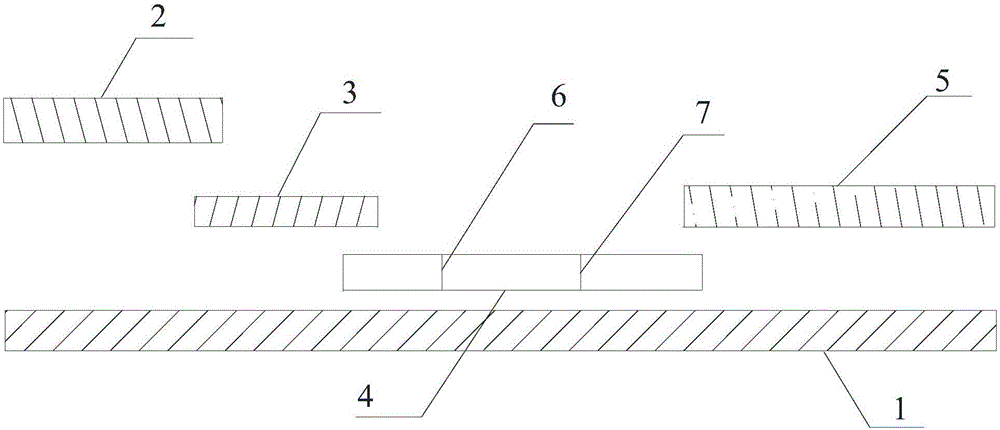
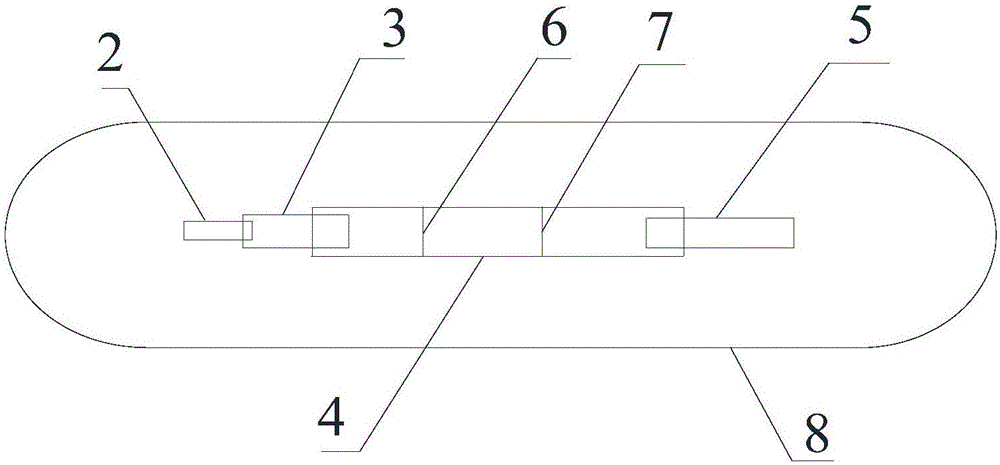
[0043] (1) Immobilizing sST2 monoclonal antibody II and rabbit anti-mouse IgG antibody recognizing different epitopes at different positions of the coating membrane 4 to form a detection line and a quality control line;
[0044] (2) prepare rare earth ion microsphere-labeled sST2 monoclonal antibody I, and spray on the binding pad 3;
[0045] (3) Paste the sample pad 2 , binding pad 3 , coating film 4 and absorbent paper 5 on the bottom plate 1 in sequence, and then cut them into 0.5 cm width and put them into the clamping case 8 .
[0046] The preparation method of coating film 4 in the step (1) is: use the phosphate buffer saline solution that the pH of 7.2 is 7.2 to use the 0.01mol / L that contains 1% sucrose, the sST2 monoclonal antibody II that recognizes different epitopes and the rabbit anti-mouse The IgG antibody was d...
Embodiment 2
[0049] The preparation method of the time-resolved fluorescent immunoassay test strip for quantitatively detecting sST2 comprises the following steps:
[0050] (1) Immobilizing sST2 monoclonal antibody II and rabbit anti-mouse IgG antibody recognizing different epitopes at different positions of the coating membrane 4 to form a detection line and a quality control line;
[0051] (2) prepare rare earth ion microsphere-labeled sST2 monoclonal antibody I, and spray on the binding pad 3;
[0052] (3) Paste the sample pad 2 , binding pad 3 , coating film 4 and absorbent paper 5 on the bottom plate 1 in sequence, and then cut them into 0.5 cm width and put them into the clamping case 8 .
[0053] The preparation method of coating film 4 in the step (1) is: use the phosphate buffer saline solution that the pH of 7.4 is 7.4 to use the 0.03mol / L containing 5.5% sucrose, respectively will recognize the sST2 monoclonal antibody II of different epitopes and the rabbit anti-mouse Dilute t...
Embodiment 3
[0056] The preparation method of the time-resolved fluorescent immunoassay test strip for quantitatively detecting sST2 comprises the following steps:
[0057] (1) Immobilizing sST2 monoclonal antibody II and rabbit anti-mouse IgG antibody recognizing different epitopes at different positions of the coating membrane 4 to form a detection line and a quality control line;
[0058] (2) prepare rare earth ion microsphere-labeled sST2 monoclonal antibody I, and spray on the binding pad 3;
[0059] (3) Paste the sample pad 2 , binding pad 3 , coating film 4 and absorbent paper 5 on the bottom plate 1 in sequence, and then cut them into 0.5 cm width and put them into the clamping case 8 .
[0060] The preparation method of coating film 4 in the step (1) is: use the phosphate buffer saline solution that the pH of 7.6 is 7.6 to use the 0.05mol / L containing 10% sucrose, the sST2 monoclonal antibody II that recognizes different epitopes and the rabbit anti-mouse The IgG antibody was dil...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com