A method for producing rare earth fluoride from bastnaesite
A technology of biscarbonate lanthanum and rare earth fluoride, which is applied in the field of rare earth production and can solve problems such as high cost, time-consuming, and non-environmental protection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
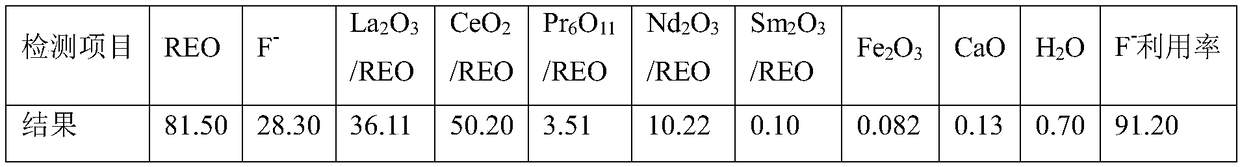
[0022] a. Decarburize and oxidize Sichuan Mianning magnetic separation ore in a muffle furnace at 500°C for 1 hour. The burnt rare earth oxide ore is ready for use. After testing, REO: 84.5%, F: 8.98%.
[0023] b. Add 700 mL of clear water to a 2000 mL beaker, add 31% industrial hydrochloric acid to prepare a hydrochloric acid solution with a concentration of 3.60 mol / L, and add 10 mL of complexing agent.
[0024] c. Add 200g of rare earth oxidized ore to be used while stirring, and continue to react for 1 hour after adding the rare earth oxidized ore, and the whole process lasts for 1.5 hours.
[0025] d. After the reaction, add 2mL of flocculant for solid-liquid separation, and obtain fluorine-containing rare earth feed liquid and a small part of leaching residue. The fluorine-containing rare earth liquid enters the next step, and the leaching residue is washed to recover rare earth. After drying, the leaching residue weighs 37g , which contains REO: 15.50%, and the rare ear...
Embodiment 2
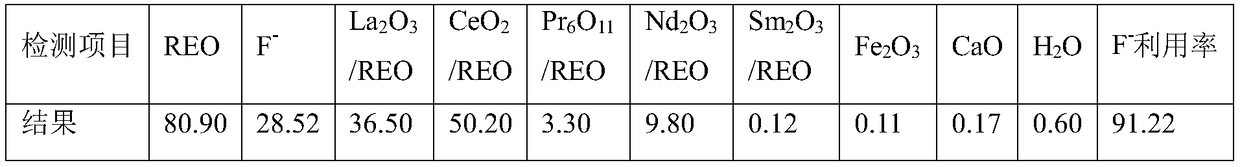
[0030] a. Decarbonize and oxidize Sichuan Mianning flotation ore in a muffle furnace at 520°C for 1.5 hours. The burned rare earth oxide ore is ready for use. After testing, REO=77.80%, F=8.30%.
[0031] b. Add 700mL of clear water to a 2000mL beaker, add 31% industrial hydrochloric acid to prepare a hydrochloric acid solution with a concentration of 3.50mol / L, and add 8mL of complexing agent.
[0032] c. Add 200g of rare earth oxidized ore to be used while stirring, and continue to react for 1 hour after adding the rare earth oxidized ore, and the whole process lasts for 1.5 hours.
[0033] d. After the reaction, add 2mL of flocculant for solid-liquid separation, and obtain fluorine-containing rare earth feed liquid and a small part of leached slag. The fluorine-containing rare earth liquid enters the next step, and the leached slag is washed to recover rare earth. After drying, the leached slag weighs 58g , which contains REO=13.41%, and the rare earth leaching rate is 95.90...
Embodiment 3
[0038] a. The Sichuan Dechang flotation ore was decarburized and oxidized by burning in a muffle furnace at 480°C for 1 hour, and the burnt rare earth oxide ore was ready for use. After testing, REO=70.53%, F=7.55%.
[0039] b. Add 700mL of clear water to a 2000mL beaker, add 31% industrial hydrochloric acid to prepare a hydrochloric acid solution with a concentration of 3.30mol / L, and add 5mL of complexing agent.
[0040] c. Add 200g of rare earth oxidized ore to be used while stirring, and continue to react for 1 hour after adding the rare earth oxidized ore, and the whole process lasts for 1.5 hours.
[0041] d. After the reaction, add 2mL flocculant for solid-liquid separation, and obtain fluorine-containing rare earth feed liquid and a small part of leaching residue. The fluorine-containing rare earth liquid enters the next step, and the leaching residue is washed to recover rare earth. After drying, the leaching residue weighs 77g , which contains REO=10.06%, and the rar...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com