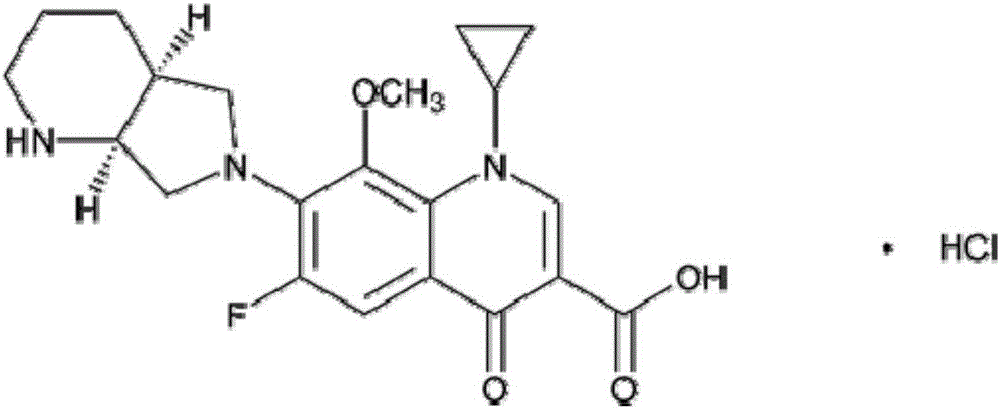
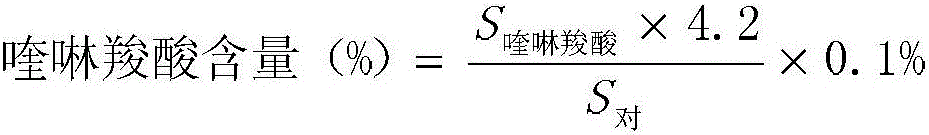Moxifloxacin hydrochloride injection pharmaceutical composition and preparation method and quality control method thereof
A technology for moxifloxacin hydrochloride and injection, which is applied in the field of quality control of moxifloxacin hydrochloride injection pharmaceutical composition, and can solve problems such as poor stability, active ingredient adsorption, slight opalescence, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0153] Embodiment 1: preparation moxifloxacin hydrochloride injection
[0154] prescription:
[0155] Moxifloxacin hydrochloride (calculated as moxifloxacin) 400mg,
[0156] Aspartic Acid 100mg,
[0157] Edetate Calcium Sodium 10mg,
[0158] The acid-base regulator adjusts the pH of the injection in an appropriate amount,
[0159] Appropriate amount of water for injection, up to 20ml.
[0160] Preparation method:
[0161] (a) Dissolve aspartic acid and calcium sodium edetate with 70% water for injection (water temperature 70° C.) of the total amount of the dosing solution, adjust the pH value of the gained medicinal solution with an acid-base regulator to be 4.5, and wait for the temperature of the medicinal solution Add moxifloxacin hydrochloride to it at 60°C, stir to dissolve;
[0162] (b) Add activated carbon (0.1% w / v by solution volume) to the obtained medicinal solution when the temperature of the medicinal solution is 40-70° C., stir and adsorb for 30 minutes, ...
Embodiment 2
[0165] Embodiment 2: preparation moxifloxacin hydrochloride injection
[0166] prescription:
[0167] Moxifloxacin hydrochloride (calculated as moxifloxacin) 350mg,
[0168] Aspartic Acid 80mg,
[0169] Edetate Calcium Sodium 12mg,
[0170] The acid-base regulator adjusts the pH of the injection in an appropriate amount,
[0171] Appropriate amount of water for injection, up to 20ml.
[0172] Preparation method:
[0173] (a) Dissolve aspartic acid and calcium sodium edetate with 60% water for injection (water temperature 75° C.) of the total amount of the dosing solution, adjust the pH value of the gained medicinal solution with an acid-base regulator to be 4.3, and wait for the temperature of the medicinal solution Add moxifloxacin hydrochloride to it at 70°C, stir to dissolve;
[0174] (b) Add active carbon (0.125% w / v by solution volume) to the obtained medicinal solution when the temperature of the medicinal solution is 40-70° C., stir and adsorb for 40 minutes, an...
Embodiment 3
[0177] Embodiment 3: preparation moxifloxacin hydrochloride injection
[0178] prescription:
[0179] Moxifloxacin hydrochloride (calculated as moxifloxacin) 450mg,
[0180] Aspartic Acid 120mg,
[0181] Edetate Calcium Sodium 8mg,
[0182] The acid-base regulator adjusts the pH of the injection in an appropriate amount,
[0183] Appropriate amount of water for injection, up to 20ml.
[0184] Preparation method:
[0185] (a) Dissolve aspartic acid and calcium sodium edetate with 80% water for injection (water temperature 60° C.) of the total amount of the dosing solution, adjust the pH value of the gained medicinal solution with an acid-base regulator to be 4.7, and wait for the temperature of the medicinal solution Add moxifloxacin hydrochloride to it at 40°C, stir to dissolve;
[0186] (b) Add activated carbon (0.075% w / v based on solution volume) to the obtained medicinal solution when the temperature of the medicinal solution is 40-70°C, stir and absorb for 20 minu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com