Method for preparing high-stability solid arsenic minerals by hydrothermal process
A high stability, hydrothermal technology, applied in the field of mineral arsenic fixation, to achieve the effect of small BET specific surface area, low equipment requirements, and wide stable area
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] The main components in the arsenic solution include: As 60g / L, Sb 1.1g / L, Pb 235ppm, Sn 38.5ppm, Te24.4ppm, NaOH 30g / L.
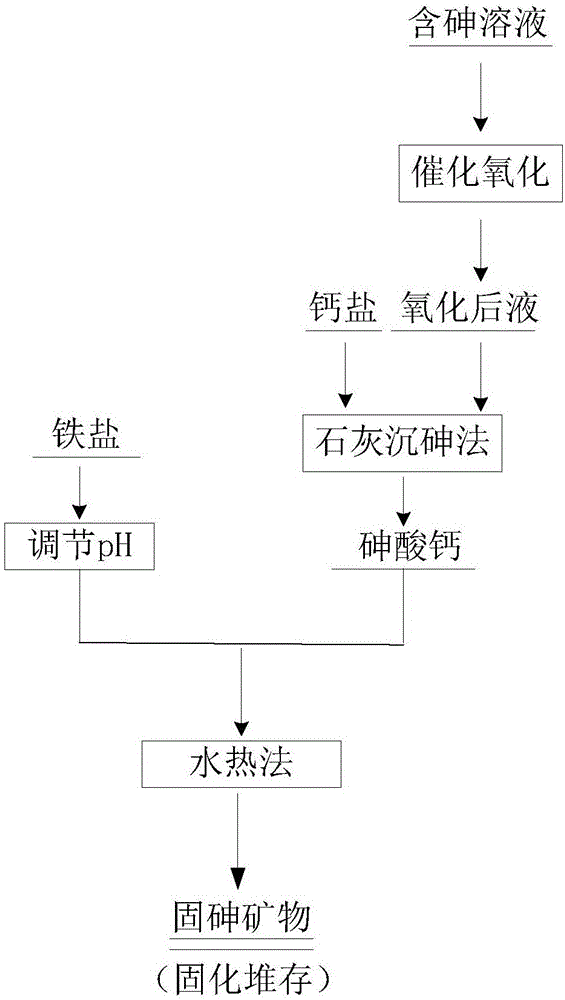
[0038] A kind of preparation method of highly stable arsenic-fixed mineral, comprises the following steps: arsenic-containing solution adopts the method for catalytic oxidation to As 3+ Oxidized to As 5+ , the control conditions are, the oxygen flow rate is 5L / min, adding KMnO 4 As a catalyst, the molar ratio of As / Mn is controlled at 10:1, and the temperature of the catalytic oxidation system is controlled at 90°C. The results show that As 3+ The conversion rate was 98.45%.
[0039] Adjust the pH value of the oxidized solution to 1.5, and add CaO and Ca(OH) in a continuous feeding mode 2 As an arsenic precipitating agent, the Ca / As molar ratio is 5, the settling time is 6 hours, and the reaction temperature is 60° C. to generate calcium arsenate. The synthesis of arsenic-fixing minerals adopts the hydrothermal method, and the control conditions ...
Embodiment 2
[0041] The main components in the arsenic solution include: As 76g / L, Sb 0.8g / L, Pb 96.7ppm, Sn 27.5ppm, Te38.7ppm, NaOH 25g / L.
[0042] A method for preparing a highly stable arsenic-fixing mineral, comprising the following steps: the arsenic-containing solution adopts a catalytic oxidation method to convert As 3+ Oxidized to As 5+ , the control conditions are, oxygen flow rate is 10L / min, adding KMnO 4 As a catalyst, the molar ratio of As / Mn is controlled at 40:1, and the temperature of the catalytic oxidation system is controlled at 30°C. The results show that As 3+ The conversion rate is 92.31%;
[0043] Adjust the pH value of the oxidized solution to 2, and add CaO and Ca(OH) in a continuous feeding mode 2 As the arsenic precipitating agent, the Ca / As molar ratio is 8, the settling time is 8 hours, and the reaction temperature is 90° C. to generate calcium arsenate. The synthesis of arsenic-fixing minerals adopts the hydrothermal method, and the control conditions are...
Embodiment 3
[0045] The main components in the arsenic solution include: As 50g / L, Sb 1.8g / L, Pb 186ppm, Sn 34.7ppm, Te27.8ppm, NaOH 5g / L.
[0046] A method for preparing a highly stable arsenic-fixing mineral, comprising the following steps: the arsenic-containing solution adopts a catalytic oxidation method to convert As 3+ Oxidized to As 5+ , the control conditions are, oxygen flow rate is 1L / min, adding KMnO 4 As a catalyst, the molar ratio of As / Mn is controlled at 20:1, and the temperature of the catalytic oxidation system is controlled at 120°C. The results show that As 3+ The conversion rate is 89.36%
[0047] Adjust the pH value of the oxidized solution to 1.5, and add CaO and Ca(OH) in a continuous feeding mode 2 As an arsenic precipitating agent, the Ca / As molar ratio is 5, the settling time is 10 hours, and the reaction temperature is 60° C. to generate calcium arsenate. The synthesis of arsenic-fixing minerals adopts the hydrothermal method, and the control conditions are ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com