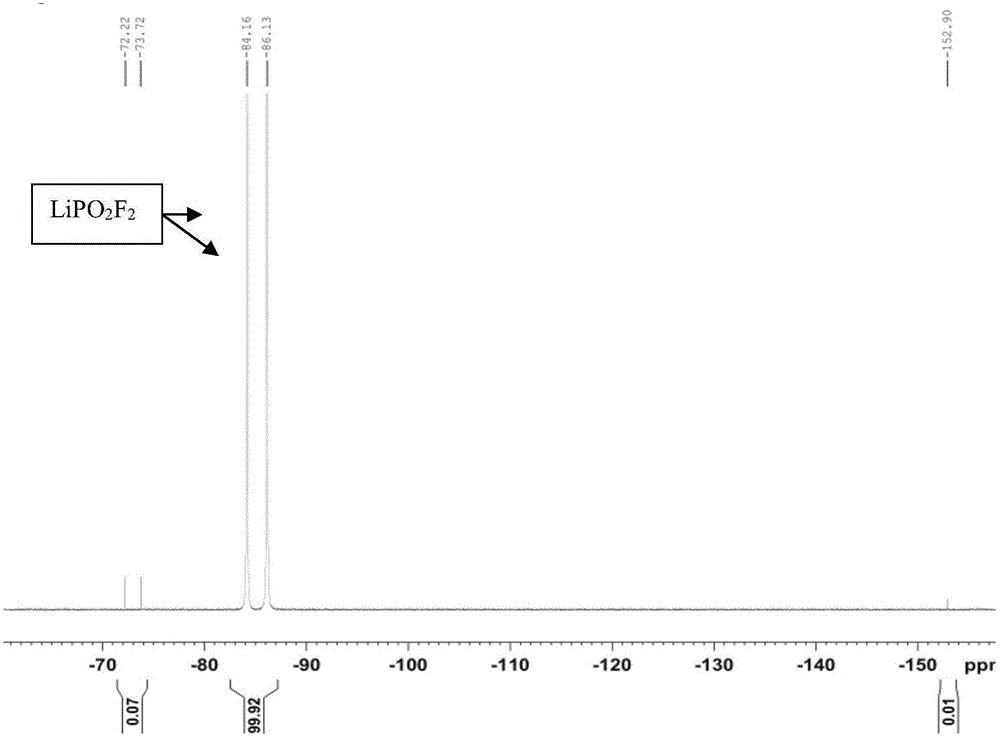
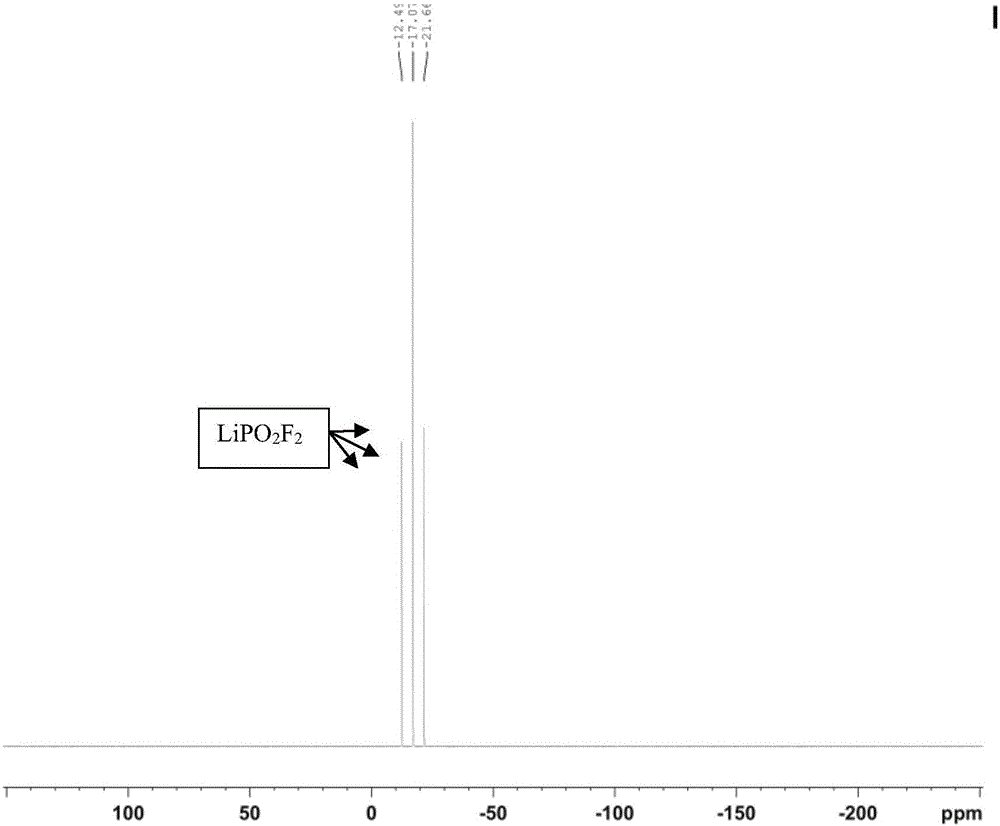
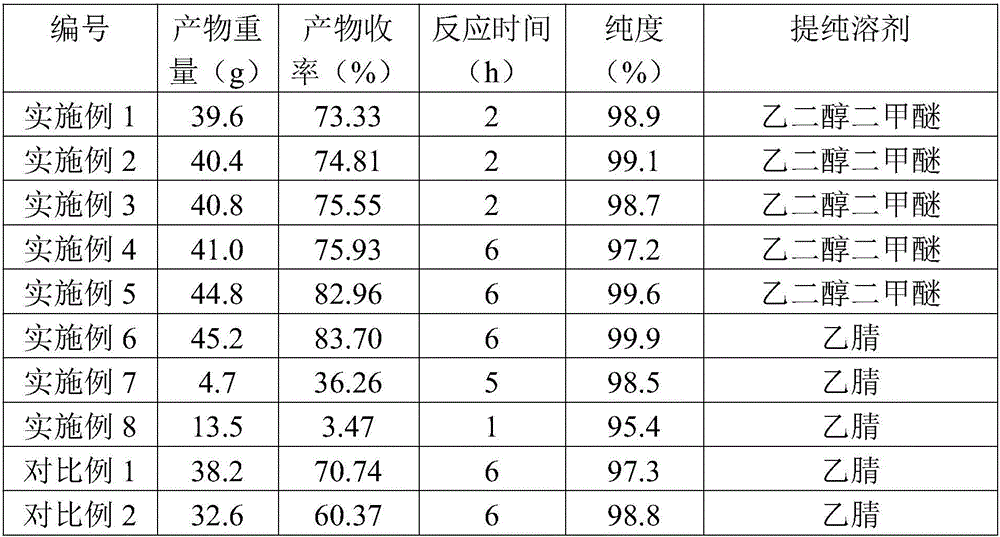
Preparation method of lithium difluorophosphate
A technology of lithium difluorophosphate and lithium carbonate, applied in chemical instruments and methods, lithium halides, phosphorus compounds, etc., can solve problems such as difficult separation and purification, complicated process, and numerous by-products, and achieve good economic value and social Value, easy promotion and application, and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] (1)LiPO 2 f 2 Synthesis of DEC: In a glove box environment, add 600mL of DEC to a 1L PFA container, and at the same time turn on the magnetic stirrer to make the stirring bar in the PFA container start to work, the stirring speed is 250r / min, and then add 1mol of lithium carbonate to make It forms a suspension.
[0054] (2) Turn on the high and low temperature circulation device, heat the PFA container, control the temperature at 62°C, and start adding 0.5mol LiPF slowly 6 , The feeding time is 10 minutes, the reaction temperature is observed during the feeding process, and the temperature is controlled below 68°C. After the feeding is completed, use the high and low temperature circulation device to raise the temperature in the PFA container to 72-73°C, and continue to stir and react for 2 hours. Observe that there is gas overflow, and the liquid is a suspension.
[0055] (3) The resulting suspension is filtered through a pressurized closed filter with a pore size ...
Embodiment 2
[0059] (1)LiPO 2 f 2 Synthesis of DEC: In a glove box environment, add 600mL of DEC to a 1L PFA container, and at the same time turn on the magnetic stirrer to make the stirring bar in the PFA container start to work, the stirring speed is 250r / min, and then add 1.2mol of lithium carbonate, to form a suspension.
[0060] (2) Turn on the high and low temperature circulation device, heat the PFA container, control the temperature at 55-65°C, and start adding 0.5mol LiPF slowly 6 , The feeding time is 10 minutes, the reaction temperature is observed during the feeding process, and the temperature is controlled below 68°C. After the feeding is completed, use the high and low temperature circulation device to raise the temperature in the PFA container to 72-73°C, and continue to stir and react for 2 hours.
[0061] (3) The resulting suspension is filtered through a pressurized closed filter with a pore size of 5 μm. The resulting filtrate is stored in container A for later use, ...
Embodiment 3
[0065] (1)LiPO 2 f 2 Synthesis of DEC: In a glove box environment, add 600mL of DEC to a 1L PFA container, and at the same time turn on the magnetic stirrer to make the stirring bar in the PFA container start to work, the stirring speed is 250r / min, and then add 1.2mol of lithium carbonate, to form a suspension.
[0066] (2) Turn on the high and low temperature circulation device, heat the PFA container, control the temperature at 55-65°C, and start adding 0.5mol LiPF slowly 6 , The feeding time is 10 minutes, the reaction temperature is observed during the feeding process, and the temperature is controlled below 68°C. After the feeding is completed, use a high and low temperature circulation device to raise the temperature in the PFA container to 75°C, and continue to stir and react for 2 hours.
[0067] (3) The resulting suspension is filtered through a pressurized closed filter with a pore size of 5 μm. The resulting filtrate is stored in container A for later use, and t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com