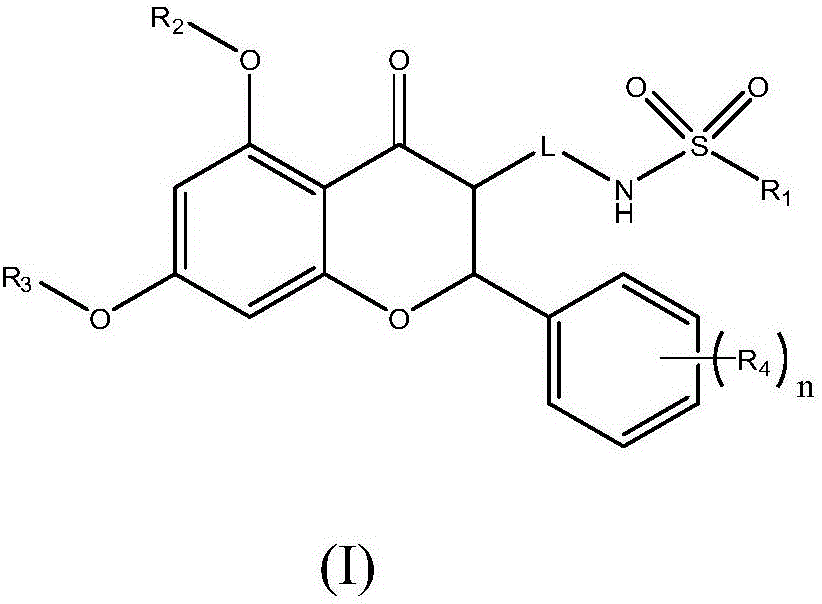
Medicine for preventing and treating cerebral arterial thrombosis and application thereof
A pharmacy and compound technology, applied in the field of medicine, can solve the problems of complex pathogenesis of ischemic cerebrovascular disease, lack of effective drugs in clinical treatment, etc., to prevent and treat ischemic stroke, reduce the percentage of the whole brain, reduce The effect of the degree of edema
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
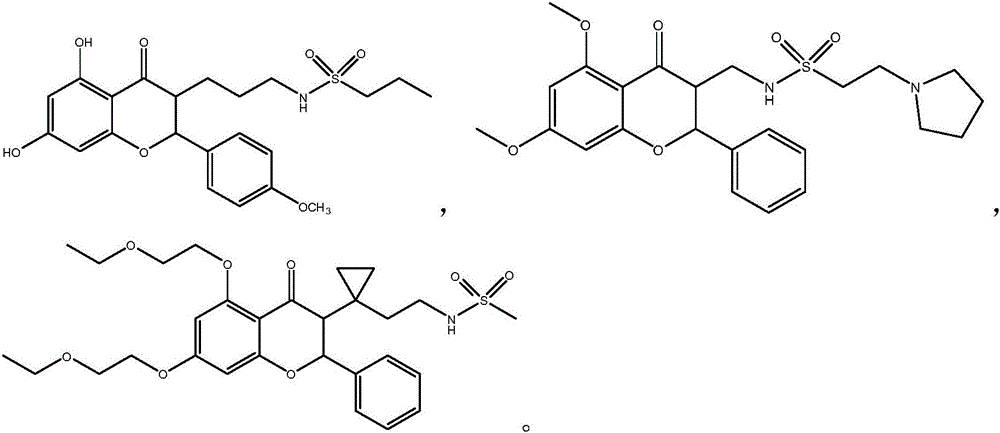
[0040] Embodiment 1: N-{3-[5,7-dihydroxyl-2-(4-methoxyphenyl)-4-oxochroman-3-yl]propyl}propane-1-sulfonamide ( Compound 1)
[0041]
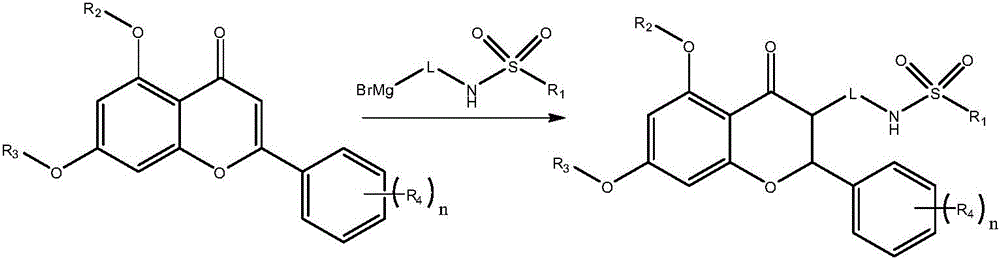
[0042] Dissolve N-(3-bromopropyl)propane-1-sulfonamide (2.9g, 12mmol) in 100mL of anhydrous tetrahydrofuran, and add new shaved magnesium chips (0.30g, 12.5mmol) dropwise under stirring, 1 grain of iodine Into the flask, first drop a small amount, and then slowly drop in after the Grignard reaction is triggered. After about 20 minutes, the dripping is completed, then stir and reflux until the magnesium chips are basically completely dissolved, stop heating, wait for the Grignard reagent to cool to 30-40°C, and add the catalyst Zirconocene dichloride (0.15g, 0.5mmol) and 5,7-dihydroxy-2-(4-methoxyphenyl)-4H-chromen-4-one (2.8g, 10.0mmol), and reflux Reacted for 2h, after the reaction was completed, the reaction solution was cooled to room temperature, and then saturated NH 4 Cl aqueous solution (100mL), extracted with petroleum ether (100mL×...
Embodiment 2
[0046] Example 2: N-[(5,7-dimethoxy-4-oxo-2-phenylchroman-3-yl)methyl]-2-(pyrroline-1-yl)ethanesulfonamide (compound 2)
[0047]
[0048] According to the method of Example 1, replace N-(3-bromopropyl) propane-1-sulfonamide with N-(bromomethyl)-2-(pyrroline-1-yl)ethanesulfonamide, and use 5, 7-dimethoxy-2-phenyl-4H-chromen-4-one in place of 5,7-dihydroxy-2-(4-methoxyphenyl)-4H-chromen-4-one gives Light yellow solid N-[(5,7-dimethoxy-4-oxo-2-phenylchroman-3-yl)methyl]-2-(pyrrolin-1-yl)ethanesulfonamide, Yield 72%.
[0049] ESI-MS: 475.18[M+H] +
[0050] Elemental analysis: theoretical value / measured value, C (60.74 / 60.89), H (6.37 / 6.27), N (5.90 / 5.81), O (20.23 / 20.33), S (6.76 / 6.80).
Embodiment 3
[0051] Example 3: N-(2-{1-[5,7-bis(2-ethoxyethoxy)-4-oxo-2-phenylchroman-3-yl]cyclopropyl}ethyl ) methanesulfonamide (compound 3)
[0052]
[0053] According to the method of Example 1, N-[2-(1-bromocyclopropyl)ethyl]methanesulfonamide is used to replace N-(3-bromopropyl)propane-1-sulfonamide, and 5,7-bis (2-Ethoxyethoxy)-2-phenyl-4H-chromene-4-one instead of 5,7-dihydroxy-2-(4-methoxyphenyl)-4H-chromene-4 -ketone, giving N-(2-{1-[5,7-bis(2-ethoxyethoxy)-4-oxo-2-phenylchroman-3-yl]cyclopropyl} as a white solid Ethyl)methanesulfonamide, 52% yield.
[0054] ESI-MS: 562.24[M+H] +
[0055] Elemental analysis: theoretical value / measured value, C (62.01 / 62.12), H (7.00 / 6.87), N (2.49 / 2.58), O (22.79 / 22.73), S (5.71 / 5.60).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com