Smoothened receptor ligand and application thereof
A technology for smoothing receptors and ligands, which can be used in pharmaceutical formulations, medical preparations containing active ingredients, organic active ingredients, etc. It can solve problems such as not pointing out the role of substituent groups in improving the thermal stability of target proteins, and achieve enhanced interaction effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
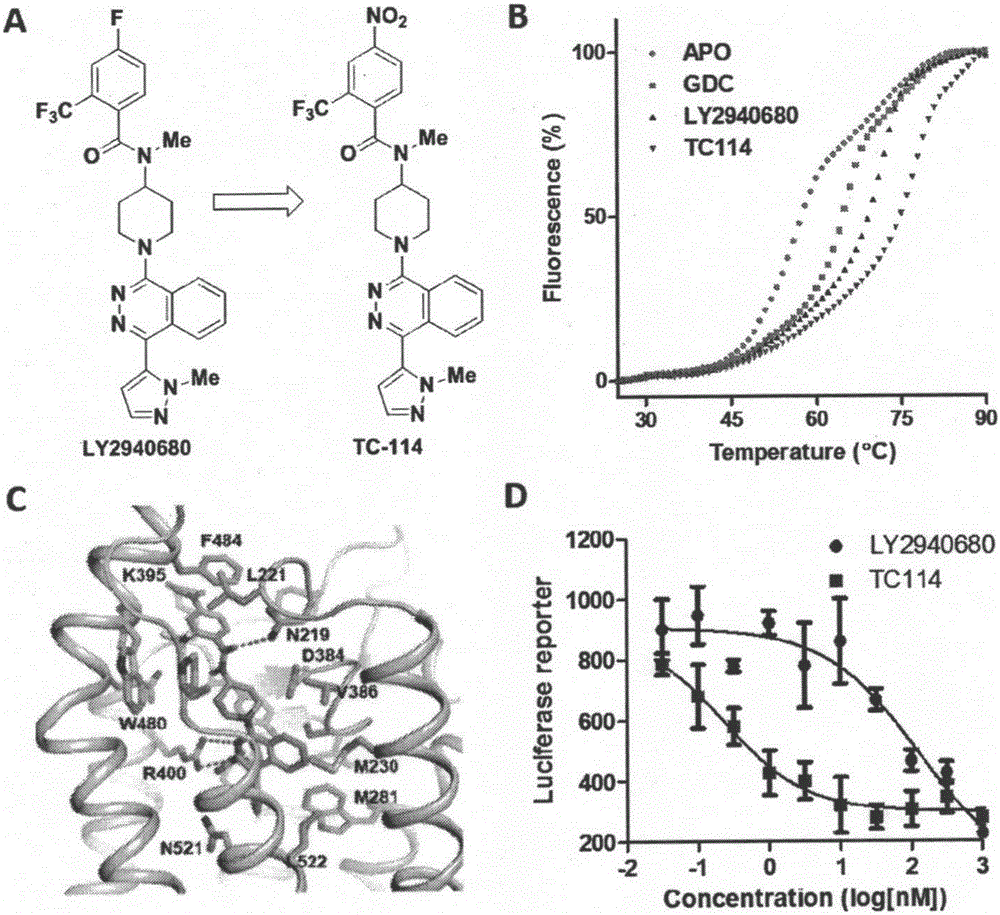
[0026] Embodiment 1: Ligand and its synthesis
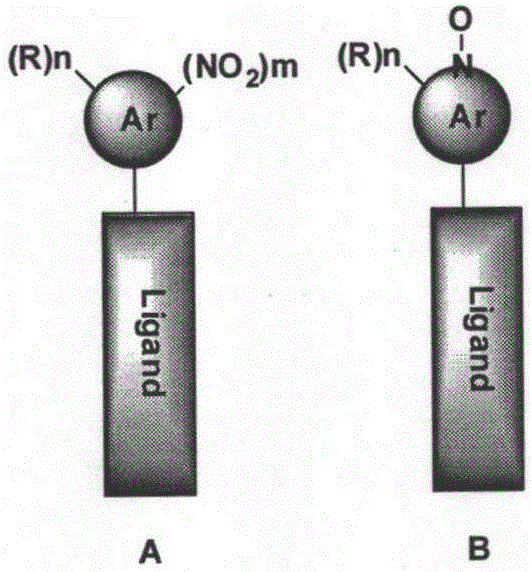
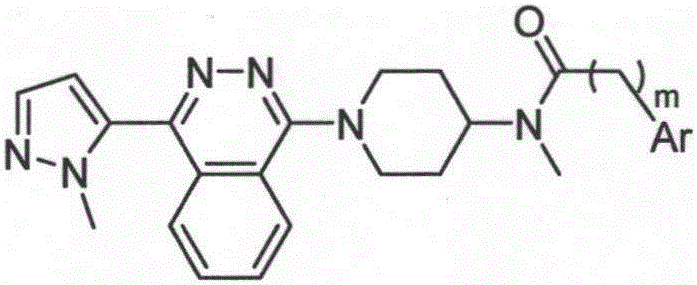
[0027] 1. Smoothened receptor ligands, the structural formulas of which are (I)-(XXIX).
[0028] 2. Except for ligands TC113, TC641, TC642, TC643, TC658 and TC659, other ligand synthesis methods are as follows:
[0029] Add 0.21g of 4-(N-Boc-methylamino)piperidine, 0.2g of 1,4-dichlorooxazine and 0.28g of potassium carbonate to 5mL of N-methylpyrrolidone (NMP), and heat to 80°C for 6h. Add 20mL saturated ammonium chloride solution after the reaction to quench, separate the organic phase, extract the water phase with dichloromethane three times, merge into the organic phase, wash with saturated ammonium chloride aqueous solution and saturated brine successively, and then wash with anhydrous sulfuric acid Dry over sodium, filter to remove sodium sulfate, and concentrate to obtain crude product. The crude product was separated by column chromatography (200-300 mesh silica gel, eluent: n-hexane:ethyl acetate=3:1) to obtain pure int...
Embodiment 2
[0100] Each ligand synthesized in Example 1 was subjected to thermostability test and cell activity test respectively:
[0101] 1. Thermal stability experiment:
[0102] SMO protein purification:
[0103] SMO protein was expressed in HEK293F cells (Life, Cat. No. K1663). Cells were cultured at 37°C until the cell density reached 1.0-1.3x10 6 cells / mL were collected, broken to obtain cell membranes, and suspended in 10 mM 4-(2-hydroxyethyl)piperazine-1-ethanesulfonic acid N-(2-hydroxyethyl)piperazine-N′-(2-ethane Sulfonic acid) (Sigma-Aldrich, product number H3375), 10mM magnesium chloride (Sigma-Aldrich, M4880), 20mM potassium chloride (Sigma-Aldrich, P9541) pH 7.5 buffer. Sodium chloride with a concentration of 1M was added to the above buffer solution to obtain a high-salt buffer solution, and sodium chloride with a concentration of 200mM was added to obtain a low-salt buffer solution. The cell membrane was washed with high-salt buffer (50 mL*3 times) and centrifuged, th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com