High-affinity peptide for tumor necrosis factor alpha and application of high-affinity peptide
A tumor necrosis factor, affinity technology, applied in the direction of medical preparations, peptides, depsipeptides containing active ingredients, etc., can solve the problems of decreased drug efficacy, body damage and side effects, affecting drug efficacy, etc., to inhibit biological Active, strong affinity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0021] 1. Obtaining and transforming peptide sequences
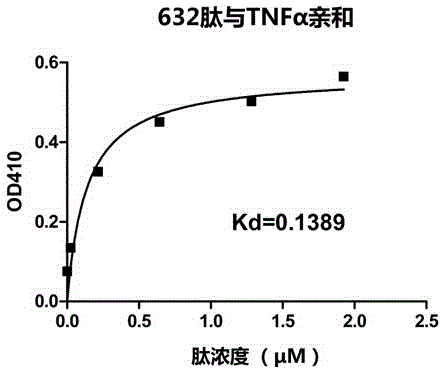
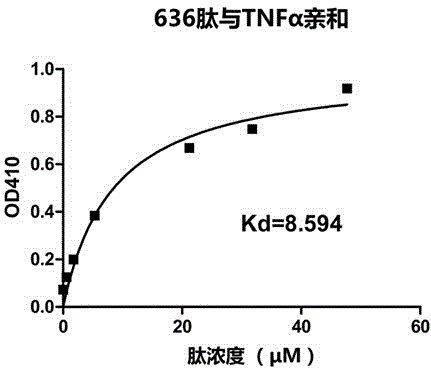
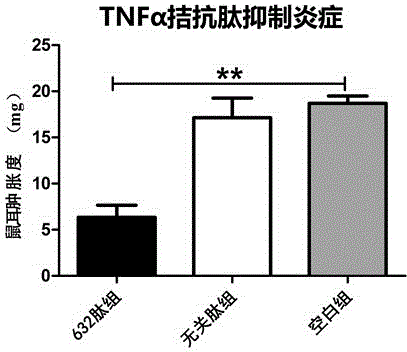
[0022] The desired peptide is artificially synthesized by chemical methods, and the peptide has an affinity with TNFα protein.
[0023] 2. Synthesis and purification of peptides
[0024] Lys(Dde)-WangResin was soaked in DCM for 10min, then drained the DCM, then added 25% piperidine (piperidine / DMF) three times the volume of the resin, blown with nitrogen for 20min, and drained the piperidine. Add DMF and blow it for 1min, and drain it after 6 cycles, and take the resin ninhydrin and detect it to be blue. The product is: H-Lys(Dde)-WangResin. Add 3 equivalents of resin to Fmoc-Val-OH, HATU, DIEA in DMF. After blowing with nitrogen for 20 minutes, drain the DMF reaction solution, add DMF and blow with nitrogen for 1 minute, then drain, repeat 3 times, and the ninhydrin detection resin becomes transparent. The product is: Fmoc-Val-Lys(Dde)-WangResin, and so on, to obtain the crude product. Purify by using acetonitrile ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com