Pharmaceutical composition for treating ulcerative colitis
A technology for ulcerative colitis and a composition, applied in the field of medicine, can solve problems such as undetermined microorganisms, and achieve the effects of relieving ulcerative colitis, fast onset, and alleviating symptoms
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
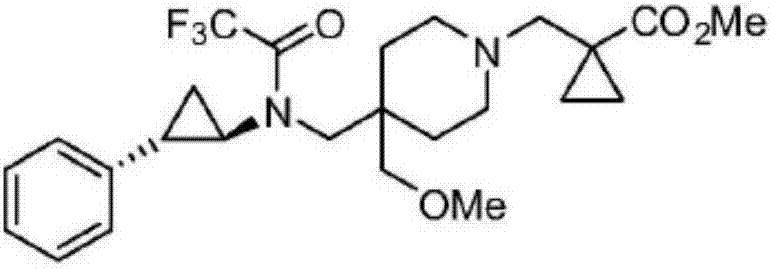
[0018] The characterization of experimental example 1 medicine of the present invention
[0019]
[0020] 1 H NMR(500MHz,DMSO)δ7.33-7.28(m,2H),7.24-7.19(m,1H),7.19-7.15(m,2H),3.40(s,2H),3.36-3.31(m,5H ),3.30-3.19(m,4H),3.14(s,2H),2.92-2.83(m,1H),2.47-2.41(m,1H),1.92-1.71(m,4H),1.54-1.41(m ,1H), 1.37-1.30(m,2H), 1.29-1.20(m,1H), 1.16-1.09(m,2H).
experiment example 2
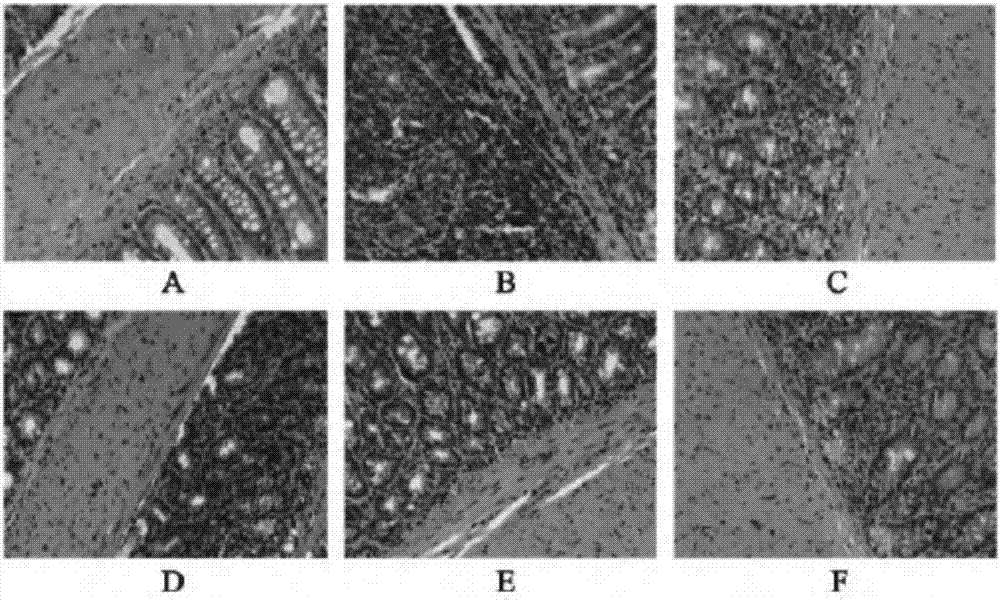
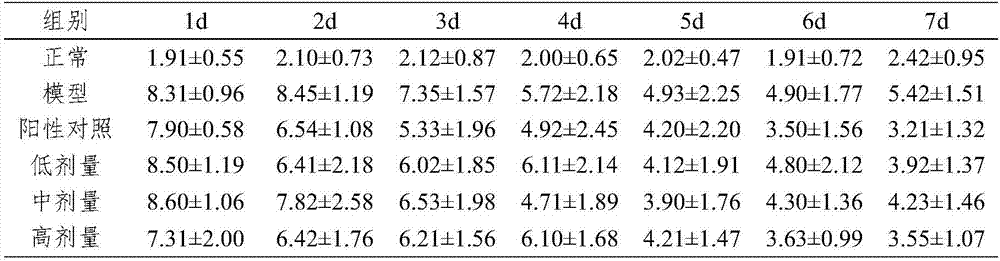
[0021] Experimental example 2 Therapeutic effect of medicine of the present invention for ulcerative colitis
[0022] SD rats were selected, and 10 of them were selected as the normal group, and the remaining rats were treated with oxazolone (OXZ)-induced ulcerative colitis (UC) model. Shave the back skin of rats (2cm×2cm), label, add 0.2mL of 3% by weight OXZ (dissolved in absolute ethanol) dropwise to the shaved place, drop continuously for 7 days, fast for 24 hours on the 7th day, and use on the 8th day Rats were anesthetized with an appropriate amount of isoflurane, and then a polyethylene tube with a diameter of 2 mm was inserted into the large intestine about 8 cm, and the volume was 1.0 mL·kg -1 Infuse 1.0% by weight OXZ (dissolved in 50% by weight ethanol aqueous solution), and place the rat upside down for 1 min after injection to prevent reflux. The OXZ-induced UC rat model was successfully established. Feed normally after waking up.
[0023] On the first day after...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com