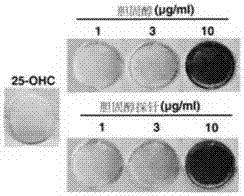
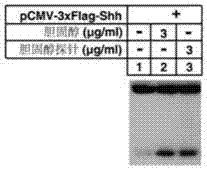
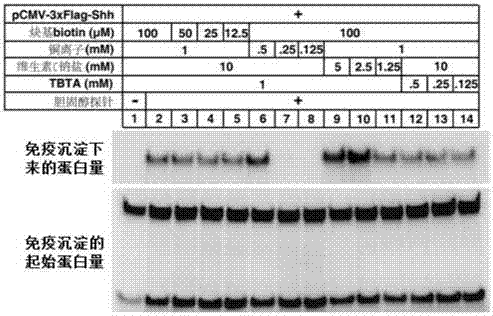
Cholesterol molecular probe as well as preparation method and application thereof
A technology of molecular probes and cholesterol, applied in biochemical equipment and methods, chemical instruments and methods, peptide sources, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0109] Embodiment 1: the preparation of compound L1
[0110] Lithocholic acid (1.88g, 5.0mmol) was dissolved in anhydrous methanol, and p-toluenesulfonic acid (100mg, 0.5mmol) was added. The reaction was carried out at room temperature for 4 hours. After the TLC detection reaction was complete, the reaction solution was concentrated, 150 mL of ethyl acetate was added, washed with saturated sodium bicarbonate solution and saturated sodium chloride solution respectively, dried over anhydrous sodium sulfate, concentrated, and subjected to silica gel column chromatography (PE:EA=1: 1) A white solid (1.9 g, 97%) was obtained. 1 H NMR (300MHz, CDCl 3 )δ 3.66(s,3H), 3.65–3.58(m,1H), 0.92(s,3H), 0.90(s,3H), 0.64(s,3H).
Embodiment 2
[0111] Embodiment 2: the preparation of compound L2
[0112] L1 (1.8 g, 4.6 mmol) was dissolved in anhydrous DMSO (30 mL) and anhydrous toluene (30 mL), and IBX (1.7 g, 6.0 mmoL) was added. React at room temperature for 3 hours, after the reaction is complete by TLC, add water and ethyl acetate, remove insoluble matter by suction filtration, extract the filtrate with ethyl acetate, combine the organic phases, wash with a large amount of water, wash with saturated sodium chloride solution, and dry over anhydrous sodium sulfate , concentrated, and silica gel column chromatography (PE:EA=2:1) gave a white solid (1.63g, 90%). 1 H NMR (300MHz, CDCl 3 ) δ 3.66 (s, 3H), 1.01 (s, 3H), 0.92 (d, J=6.3Hz, 3H), 0.68 (s, 3H).
Embodiment 3
[0113] Embodiment 3: the preparation of compound L3
[0114] Dissolve L2 (1.9g, 4.9mmol) in anhydrous DMSO, add IBX (1.7g, 6.0mmoL) and a catalytic amount of trifluoroacetic acid, stir at 40°C for 12 hours, after TLC detects that the reaction is complete, add water and ethyl acetate Insoluble matter was removed by suction filtration, the filtrate was extracted with ethyl acetate, the organic phases were combined, washed with a large amount of water, washed with saturated sodium chloride solution, dried over anhydrous sodium sulfate, concentrated, and obtained by silica gel column chromatography (PE:EA=2:1) White solid (855mg, 45%). 1 H NMR (300MHz, CDCl 3 )δ 5.72 (s, 1H), 3.66 (s, 3H), 1.17 (s, 3H), 0.91 (d, J=6.3Hz, 3H), 0.70 (s, 3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com