A method for restoring the capacity of an all-vanadium redox flow battery
An all-vanadium redox flow battery and capacity recovery technology, which is used in regenerative fuel cells, secondary battery repair/maintenance, secondary battery charging/discharging, etc. problem, to achieve the effect of easy operation, solving capacity attenuation, and low price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
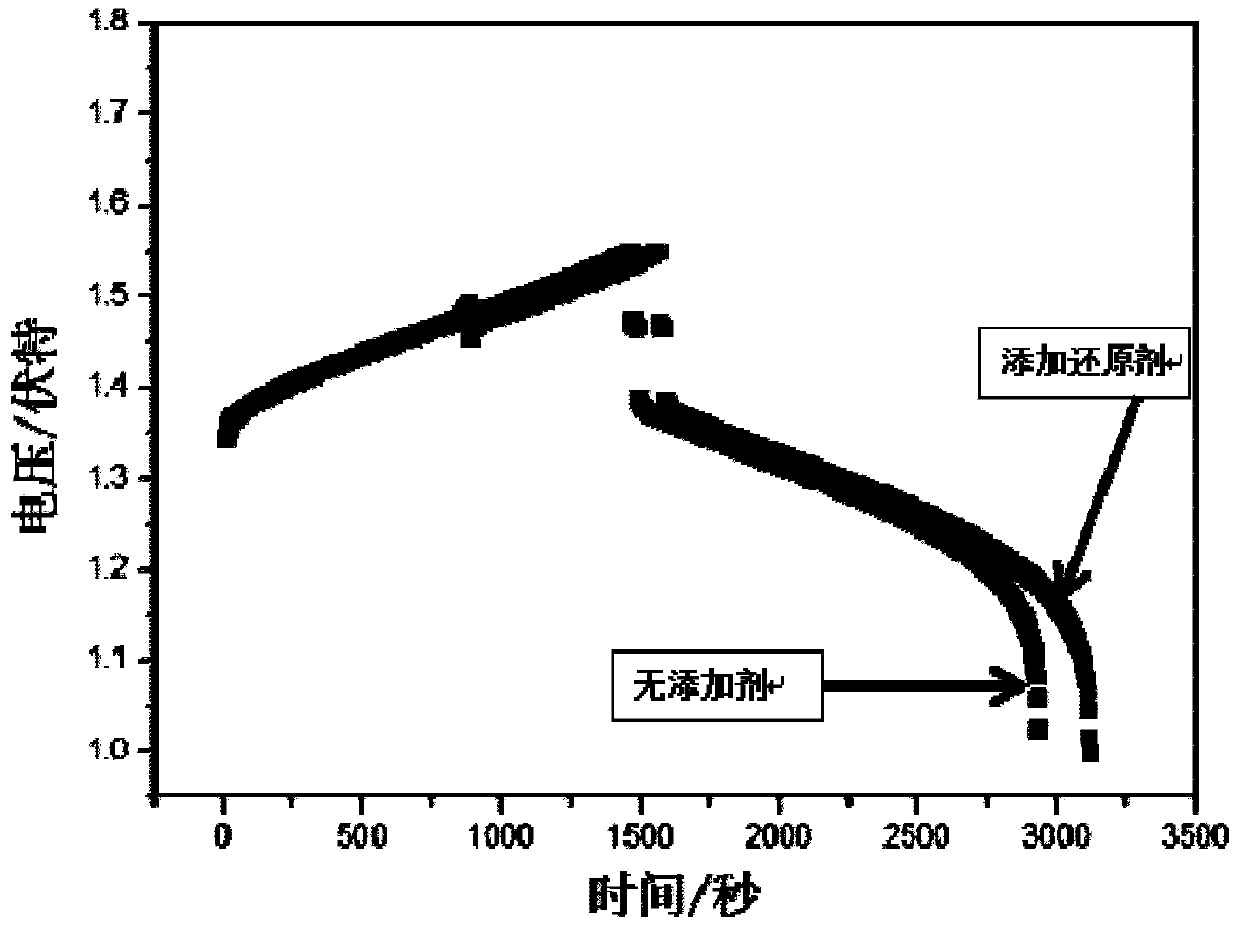
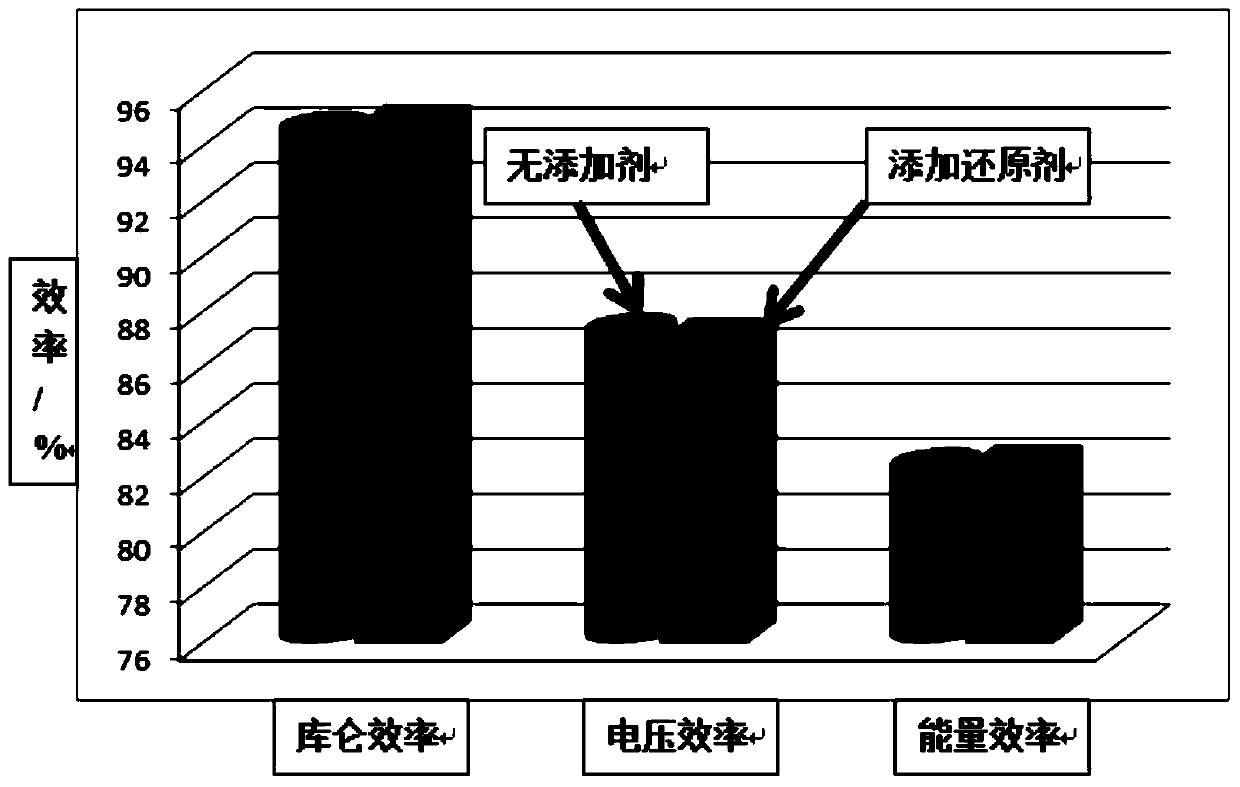
[0019] After long-term use and discharge of the battery, the battery capacity decays by more than 50%, the coulombic efficiency of the battery is 94.5%, the voltage efficiency is 87.2%, and the energy efficiency is 82.3%. The ion is 0.9mol / L, the tetravalent vanadium ion is 0.7mol / L, the sulfuric acid concentration is 3mol / L, and the electrolyte volume is 100mL; the divalent vanadium ion in the negative electrode electrolyte is 0.04mol / L, and the trivalent vanadium ion is 0.04mol / L. is 1.56mol / L, the sulfuric acid concentration is 3mol / L, and the electrolyte volume is 100mL. During the electrolyte flow process, add 2.5 grams of ferrous ammonium sulfate to the positive storage tank, the concentration of ferrous ammonium sulfate is 0.06mol / L, resume the battery charge and discharge test, the battery capacity returns to 63% of the initial capacity, and the coulombic efficiency is 94.3 %, the voltage efficiency is 87.5%, and the energy efficiency is 82.5%.
Embodiment 2
[0021] After long-term use and discharge of the battery, the battery capacity decays by more than 50%, the coulombic efficiency of the battery is 94.5%, the voltage efficiency is 87.2%, and the energy efficiency is 82.3%. The ion is 0.9mol / L, the tetravalent vanadium ion is 0.7mol / L, the sulfuric acid concentration is 3mol / L, and the electrolyte volume is 100mL; the divalent vanadium ion in the negative electrode electrolyte is 0.04mol / L, and the trivalent vanadium ion is 0.04mol / L. is 1.56mol / L, the sulfuric acid concentration is 3mol / L, and the electrolyte volume is 100mL. During the flow of the electrolyte, add 7.5 grams of ferrous ammonium sulfate to the positive storage tank, the concentration of ferrous ammonium sulfate is 0.2mol / L, resume the battery charge and discharge test, the battery capacity returns to 76% of the initial capacity, and the coulombic efficiency is 94.6 %, the voltage efficiency is 87.2%, and the energy efficiency is 82.5%.
Embodiment 3
[0023] After long-term use and discharge of the battery, the battery capacity decays by more than 50%, the coulombic efficiency of the battery is 94.5%, the voltage efficiency is 87.2%, and the energy efficiency is 82.3%. The ion is 0.9mol / L, the tetravalent vanadium ion is 0.7mol / L, the sulfuric acid concentration is 3mol / L, and the electrolyte volume is 100mL; the divalent vanadium ion in the negative electrode electrolyte is 0.04mol / L, and the trivalent vanadium ion is 0.04mol / L. is 1.56mol / L, the sulfuric acid concentration is 3mol / L, and the electrolyte volume is 100mL. During the flow of the electrolyte, add 10 grams of ferrous ammonium sulfate to the positive storage tank, the concentration of ferrous ammonium sulfate is 0.26mol / L, resume the battery charge and discharge test, the battery capacity returns to 80.7% of the initial capacity, and the coulombic efficiency is 94.7 %, the voltage efficiency is 87%, and the energy efficiency is 82.4%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| current efficiency | aaaaa | aaaaa |
| voltage efficiency | aaaaa | aaaaa |
| voltage efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com