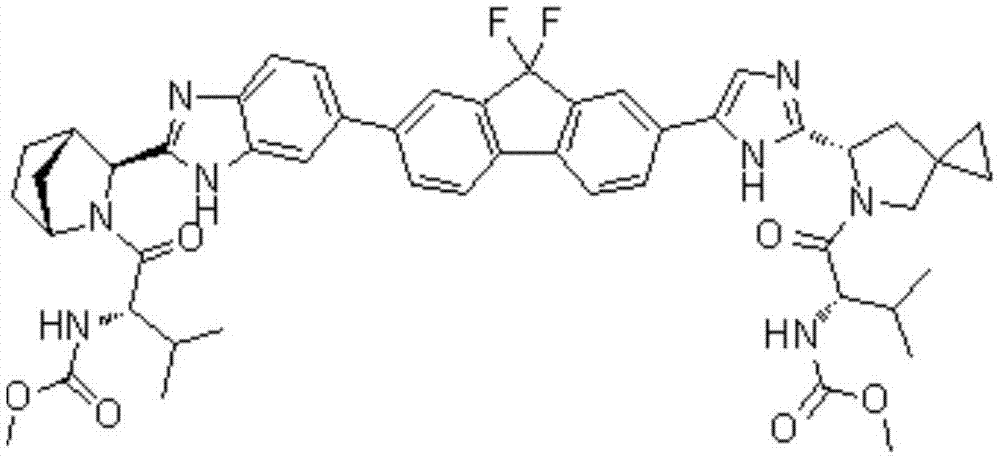
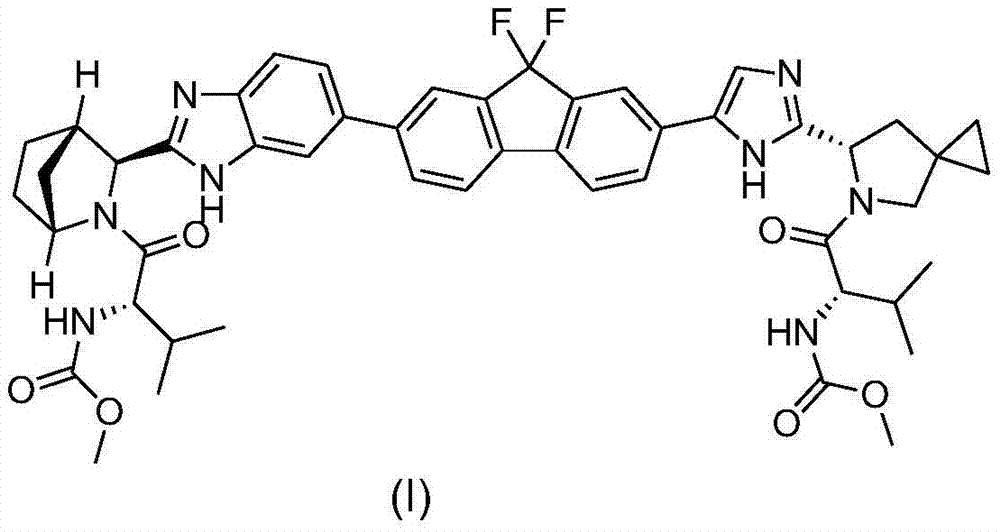
Preparation method of ledipasvir
A compound and chemical formula technology, applied in the field of drug synthesis, can solve the problems of short synthetic route, long synthetic route of ledipasvir, low reaction yield, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
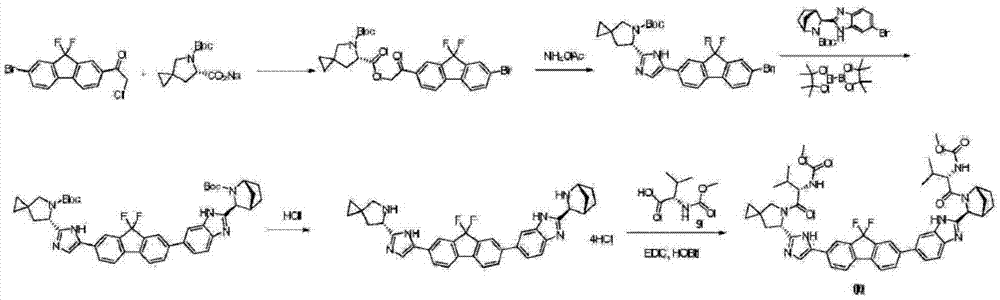
[0072] Compound (II) (30.43g, 102mmol), compound (III) (35.76g, 100mmol), and acetonitrile (360mL) were stirred uniformly in a three-necked flask, and then potassium carbonate (27.64g, 200mmol) was added and heated to 50°C for reaction After 4-6 hours, water (720 mL) was added after the reaction was completed, cooled to room temperature for beating, filtered, washed with water, and dried to obtain compound (IV) (59.47 g, yield 96%).
[0073]
[0074] After compound (IV) (59.47g, 96mmol), compound (V) (43.94g, 100mmol), tetrakistriphenylphosphine palladium (3.32g, 2.88mmol) and isopropyl acetate (600mL) were stirred and dissolved in a three-necked flask , switch the nitrogen gas under vacuum, add an aqueous deoxygenated potassium phosphate solution (2.0mol / L, 300mL), heat to 80°C and react for 6-8 hours. The organic phase was washed twice with water (300 mL), dried over sodium sulfate, and concentrated to obtain compound (VI) (85 g, crude product 100%), which was directly us...
Embodiment 2
[0084] Compound (II) (24.71g, 102mmol), compound (III) (35.76g, 100mmol), and acetonitrile (360mL) were stirred uniformly in a three-necked flask, then potassium carbonate (27.64g, 200mmol) was added, and heated to 50°C for reaction 4 After -6 hours, add water (720 mL) after the reaction is completed, cool to room temperature, beat, filter, wash with water, and dry to obtain compound (IV) (54.55 g, yield 97%).
[0085]
[0086] Compound (IV) (54.55g, 97mmol), compound (V) (44.39g, 101mmol), tetrakistriphenylphosphine palladium (3.36g, 2.91mmol) and isopropyl acetate (55mL) were stirred and dissolved in a three-necked flask Afterwards, switch the nitrogen gas under vacuum, add an aqueous solution of potassium deoxyphosphate (2.0mol / L, 300mL), heat to 80°C and react for 6-8 hours. The combined organic phases were washed twice with water (300 mL), dried over sodium sulfate, and concentrated to obtain compound (VI) (79.50 g, crude yield 100%), which was directly used for the ne...
Embodiment 3
[0096] Stir compound (II) (30.43g, 102mmol), compound (III) (35.76g, 100mmol), acetonitrile (360mL) in a three-necked flask, add potassium carbonate (27.64g, 200mmol), and heat to 50°C for reaction After 4-6 hours, water (720 mL) was added after the reaction was completed, cooled to room temperature for beating, filtered, washed with water, and dried to obtain compound (IV) (59.47 g, yield 96%).
[0097]
[0098] Compound (IV) (59.47g, 96mmol), compound (V) (49.64g, 100mmol), tetrakistriphenylphosphine palladium (3.32g, 2.88mmol) and isopropyl acetate (600mL) were stirred and dissolved in a three-necked flask Afterwards, switch the nitrogen gas under vacuum, add an aqueous solution of potassium deoxyphosphate (2.0mol / L, 300mL), heat to 80°C and react for 6-8 hours. The combined organic phases were washed twice with water (300 mL), dried over sodium sulfate, and concentrated to obtain compound (VI) (91 g, crude product 100%), which was directly used in the next reaction.
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com