Preparation method of a magnetic nano-interface catalyst with adjustable tempo loading
A magnetic nanometer, loading technology, applied in chemical instruments and methods, physical/chemical process catalysts, organic compound/hydride/coordination complex catalysts, etc. Difficult to control, complex catalyst synthesis methods, etc., to achieve the effect of simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Example 1: Preparation of Nanomagnetic Microspheres (II)
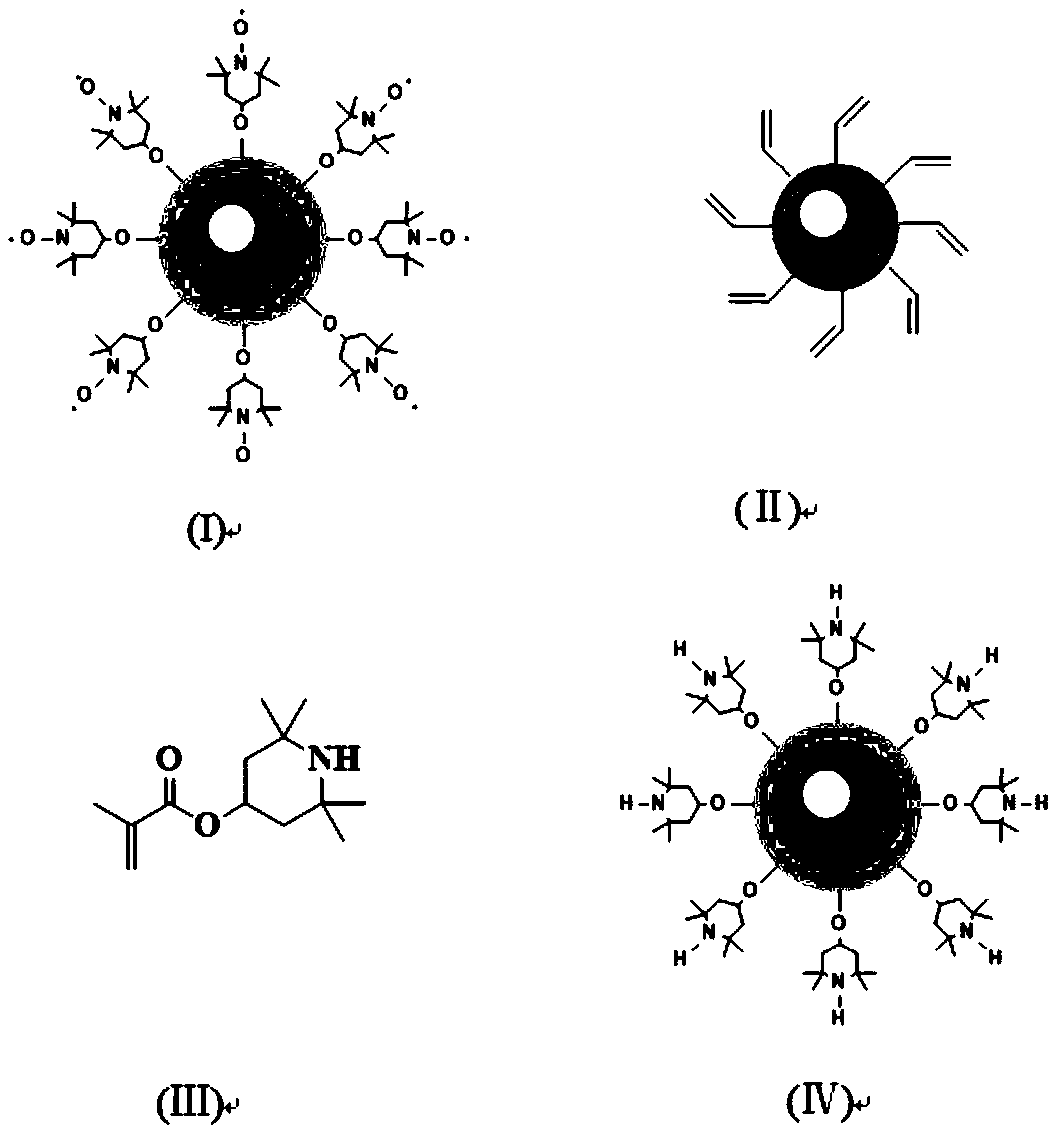
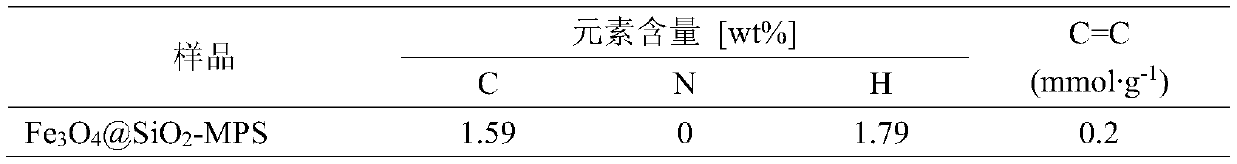
[0027] Sodium dodecylbenzenesulfonate (10.5 g, 30 mmol) was ultrasonically dispersed in 90 mL of xylene to give a clear and transparent solution. FeCl 2 ·4H 2 O (1.2 g, 6 mmol) and Fe (NO 3 ) 3 ·9H 2 O (4.85 g, 12 mmol) was dissolved in 5.4 mL of deionized water to form an iron salt solution, which was added dropwise to the xylene solution of sodium dodecyl benzene sulfonate under stirring, and stirred overnight with nitrogen for 1 h. , forming a homogeneous and stable inverse micellar emulsion. Then, the reverse micelle emulsion was heated to 70°C and kept for 3 hours. After adding 6mL of 34wt% hydrazine hydrate solution and reacting for 1 hour, the emulsion system turned black. The temperature was lowered to 30°C, and tetraethyl silicate (6 mL) and methacryloyloxypropyltrimethoxysilane (15 mL) were added for hydrolysis for 24 h. After the reaction, the emulsion was first broken with anhydrous ethanol, t...
Embodiment 2
[0029] Example 2: Preparation of Nanomagnetic Microspheres (II)
[0030] Sodium dodecylbenzenesulfonate (10.5 g, 30 mmol) was ultrasonically dispersed in 90 mL of xylene to give a clear and transparent solution. FeCl 2 ·4H 2 O (1.2 g, 6 mmol) and Fe (NO 3 ) 3 ·9H 2 O (4.85 g, 12 mmol) was dissolved in 5.4 mL of deionized water to form an iron salt solution, which was added dropwise to the xylene solution of sodium dodecyl benzene sulfonate under stirring, and stirred overnight with nitrogen for 1 h. , forming a homogeneous and stable inverse micellar emulsion. Then the reverse micelle emulsion was heated to 90° C. and kept for 1 hour. After adding 6 mL of 34 wt % hydrazine hydrate solution and reacting for 3 hours, the emulsion system turned black. The temperature was lowered to 40° C., and tetraethyl silicate (6 mL) and methacryloyloxypropyltrimethoxysilane (15 mL) were added for hydrolysis for 24 h. After the reaction, the emulsion was first broken with anhydrous etha...
Embodiment 3
[0032] Example 3: Preparation of Nanomagnetic Microspheres (II)
[0033] Sodium dodecylbenzenesulfonate (10.5 g, 30 mmol) was ultrasonically dispersed in 111.5 mL of xylene to give a clear and transparent solution. FeCl 2 ·4H 2 O (2.4 g, 12 mmol) and Fe (NO 3 ) 3 ·9H 2 O (9.7 g, 24 mmol) was dissolved in 5.4 mL of deionized water to form an iron salt solution, which was added dropwise to the xylene solution of sodium dodecyl benzene sulfonate under stirring, and stirred overnight with nitrogen for 1 h. , forming a homogeneous and stable inverse micellar emulsion. Then the reverse micelle emulsion was heated to 90° C. and kept for 1 h. After adding 6 mL of 34 wt% hydrazine hydrate solution, the reaction was performed for 3 h, and the emulsion system turned black. The temperature was lowered to 40° C., and tetraethyl silicate (16 mL) and methacryloyloxypropyltrimethoxysilane (43 mL) were added for hydrolysis for 48 h. After the reaction, the emulsion was first broken with...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com