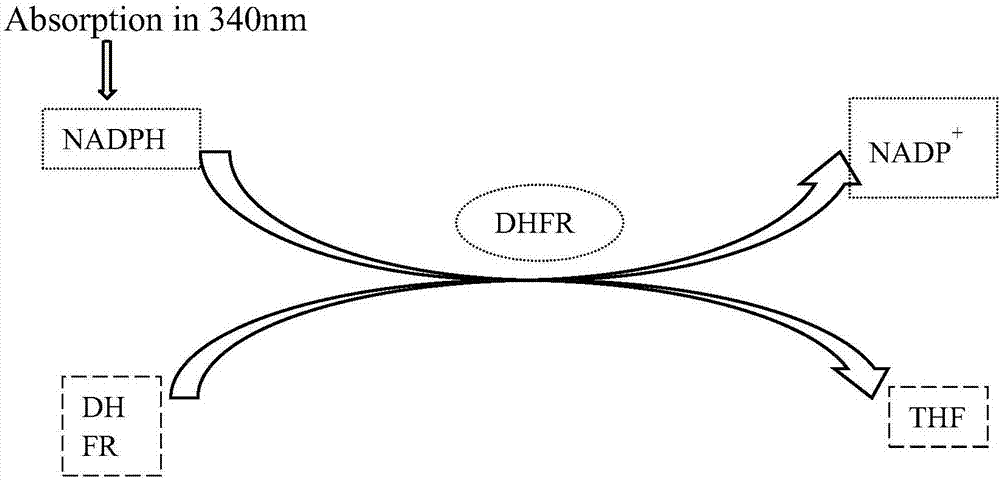
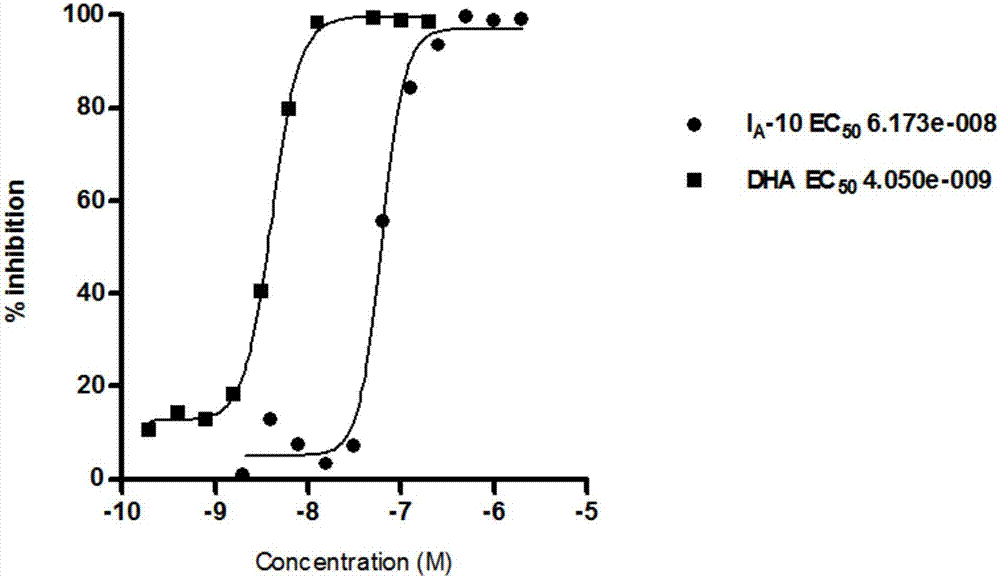
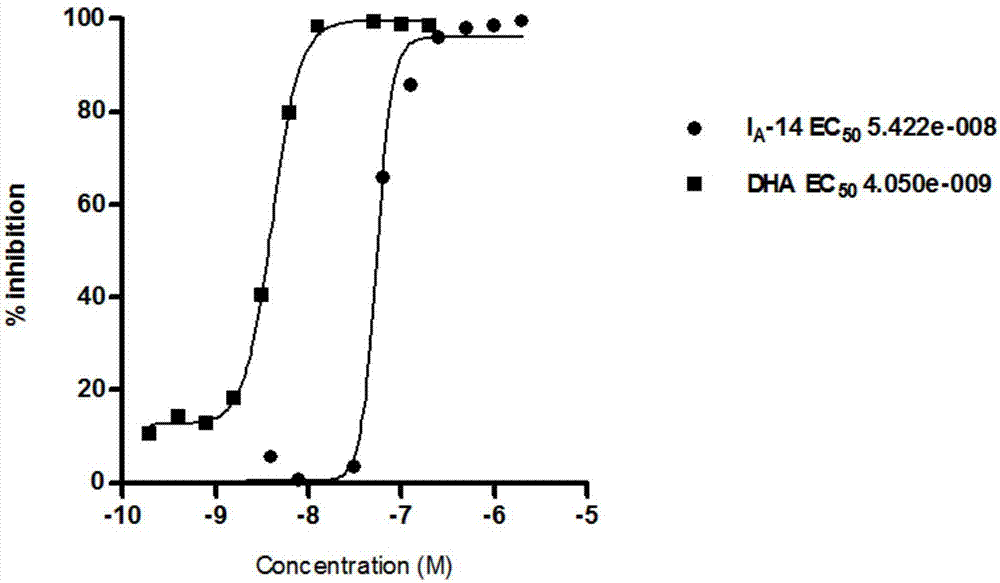
Acetamides compound and application thereof
A compound, acetamide technology, applied in the field of N-acetamide compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0045] The method for the compound shown in the preparation formula I provided by the invention specifically comprises the following steps:
[0046] (1) the carboxylic acid (R 2 COOH) was dissolved in dichloromethane and added 1-ethyl-(3-dimethylaminopropyl)carbodiimide hydrochloride and 1-hydroxybenzotriazole under stirring, reacted at room temperature for 1 hour and then added N , N-diisopropylethylamine, 4-dimethylaminopyridine, N,N-dimethylaniline, add 4-bromophenethylamine, react at room temperature for 24 hours, stop the reaction, extract with ethyl acetate , concentrated organic phase (decompression distillation to remove solvent), silica gel column chromatography purification, obtains the compound shown in formula II;
[0047] (2) Dissolve the compound represented by formula II in anhydrous tetrahydrofuran, add n-butyllithium at -78°C, react for 1 hour, then add N,N-dimethylformamide, return to room temperature, and wash with water , extracted with ethyl acetate, the...
Embodiment 1
[0053] N-(4-(((2,4-diaminoquinazolin-6-yl)amino)methyl)phenethyl)-4-nitrobenzamide (labeled "I A -1") Preparation:
[0054] (1) Preparation of N-(4-bromophenyl)-4-nitrobenzamide (compound shown in formula II-1)
[0055]
[0056] Dissolve 1.67g (10mmol) of 4-nitrobenzoic acid in 50ml of dichloromethane and add 3.8g (20mmol) of 1-ethyl-(3-dimethylaminopropyl) carbodiimide hydrochloride under stirring, 2.7g (20mmol) 1-hydroxybenzotriazole, after reacting at room temperature for 1 hour, add 2.58g (20mmol) N,N-diisopropylethylamine, 0.122g (1mmol) 4-dimethylaminopyridine, then add 2.0g (10mmol) 4-bromophenethylamine, reacted at room temperature for 24 hours, stopped the reaction, added water, extracted with ethyl acetate, concentrated the organic phase (removed solvent by distillation under reduced pressure), purified by silica gel column chromatography (elution The solution was ethyl acetate:petroleum ether=1:10, v / v), and a white solid (compound represented by formula II-1) ...
Embodiment 2
[0072] N-(4-(((2,4-diaminoquinazolin-6-yl)amino)methyl)phenethyl)-2,3-dihydrobenzo[b][1,4]dioxo Fen-6-carboxamide (marked as "I A -2") Preparation:
[0073] Except that 4-nitrobenzoic acid is replaced by 2,3-dihydrobenzo[b][1,4]dioxincarboxylic acid, all the other required raw materials, reagents and preparation methods are the same as in Example 1 to obtain a white solid ( I A -2).
[0074] 1 H NMR (400MHz, DMSO) δ8.95(t, J=7.0Hz, 1H), 8.60–8.31(m, 3H), 8.10–7.80(m, 2H), 7.50(m, 1H), 7.24(d, J=8.0Hz, 4H), 7.01(m, 5H), 6.31(t, J=5.6Hz, 1H), 4.21(d, J=5.8Hz, 2H), 3.32(dd, J=14.5, 6.5Hz, 4H), 3.17(d, J=4.8Hz, 1H), 2.84(t, J=7.5Hz, 2H).HRMS(EI) m / z calce C 26 h 26 N 6 o 3 (M + ) 470.2066 (theoretical value), experimental value (found) 470.2064.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com