Application of ginsenoside Rg5 in preparation of antidepressant drug
A ginsenoside and anti-depressant technology, applied in the field of biomedicine, can solve the problems of insufficient depression effect, intestinal and liver damage, large toxic and side effects, etc., achieve significant effect, less toxic and side effects, and reduce immobility time.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] A kind of ginsenoside Rg 5 Capsules and preparation method thereof
[0032] A kind of ginsenoside Rg 5 The capsules consist of the following raw materials:
[0033]
[0034] The preparation method of above-mentioned capsule, comprises the following steps:
[0035] 1. Weigh ginsenoside Rg according to the above prescription 5 Powder, microcrystalline cellulose and croscarmellose sodium are mixed through an 80-mesh sieve once, and the mixed powder is poured into a wet granulator;
[0036] 2. Add povidone K30 to 500ml purified water and stir to dissolve completely;
[0037] 3. Slowly pour the binder solution 2 into the wet granulator for wet granulation;
[0038] 4. Spread the prepared granules on a baking tray, put them in an oven, and dry at 50°C for 1 hour;
[0039] 5. Take out the dried granules and mix them with magnesium stearate, and pass through a 20-mesh sieve for granulation;
[0040] 6. Fill the prepared granules in 2# capsules to obtain ginsenoside Rg...
Embodiment 2
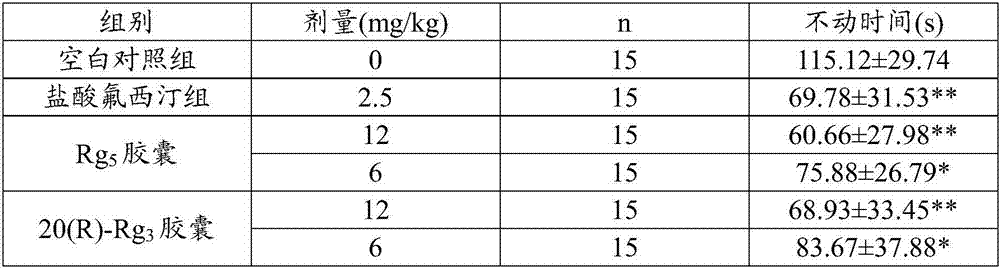
[0043] Embodiment 2 mouse forced swimming experiment
[0044] 1 Experimental materials
[0045] The Rg prepared by embodiment 1 5 capsule. 20(R)-Rg prepared by the control example 3 capsule. Fluoxetine hydrochloride capsules (Eli Lilly Suzhou Co., Ltd., specification 20mg / capsule (calculated as fluoxetine), batch number: SS41AC). ICR mice, male, weighing 18-22g. Thermometer, stopwatch, water bath, glass tank, video camera.
[0046] 2 Experimental methods
[0047]2.1 90 normal mice were divided into 6 groups on average, namely blank control group, positive drug fluoxetine hydrochloride group 2.5mg / kg, Rg 5 Capsule 12mg / kg, 6mg / kg dose group and 20(R)-Rg 3 Capsule 12mg / kg, 6mg / kg dose group. The above-mentioned capsules were dissolved in deionized water, and each group was dosed at 0.2ml / 10g body weight. Once a day, continuous administration for 7 days, the blank control group was given deionized water.
[0048] 2.2 All the above groups were administered continuously ...
Embodiment 3
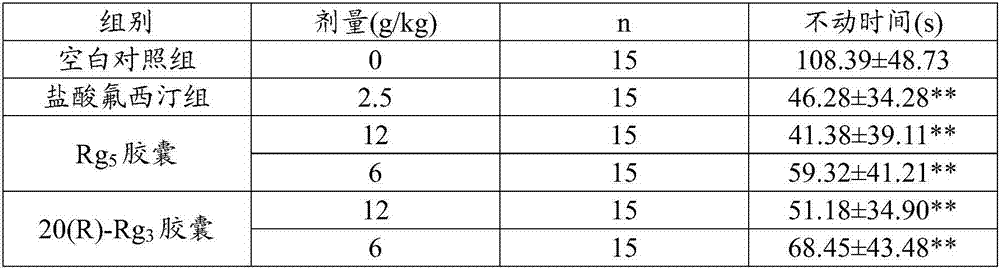
[0057] Embodiment 3 Mouse tail suspension experiment
[0058] 1 Experimental materials
[0059] The Rg prepared by embodiment 1 5 capsule. 20(R)-Rg prepared by the control example 3 capsule. Fluoxetine hydrochloride capsules (Eli Lilly Suzhou Co., Ltd., specification 20mg / capsule (calculated as fluoxetine), batch number: SS41AC). ICR mice, male, weighing 18-22g.
[0060] 2 Experimental methods
[0061] 2.1 Animal grouping and administration method, dose and time are the same as in Example 2.
[0062] 2.2 All the above groups were administered continuously for 7 days, and the experiment was carried out 1 hour after the administration on the seventh day. Fix the tail end of the mouse (2cm away from the tail tip) on the connection line of the 300g tension transducer with adhesive tape, so that it is in an upside-down state, and the head is about 15cm away from the experimental platform. Two animals are tested at the same time each time, and the separated by cardboard. Co...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com