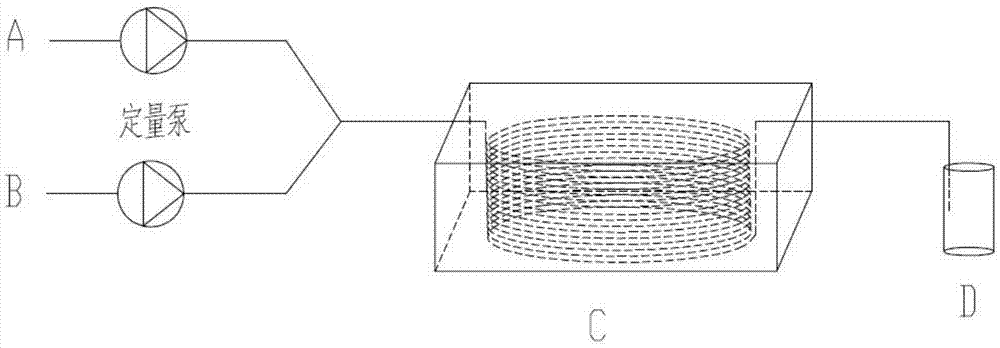
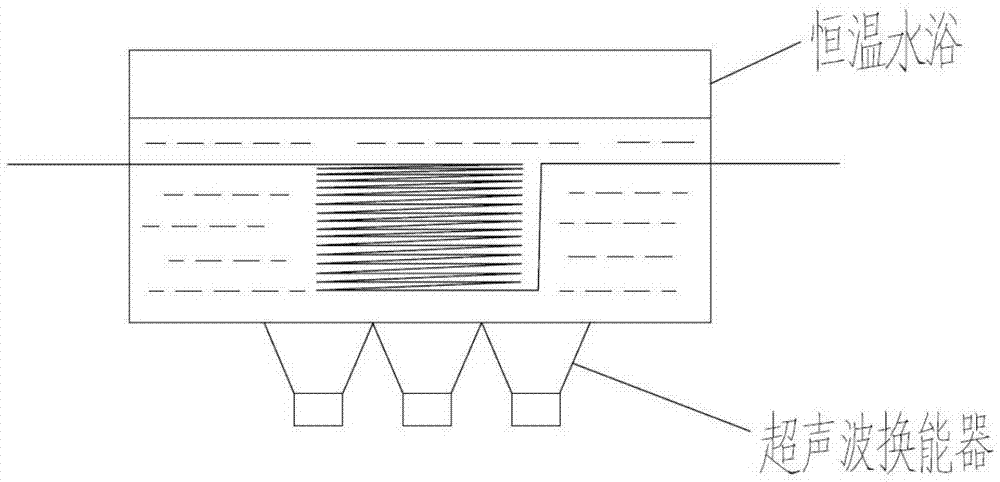
Continuous nitrification method of naphthalene sulfonate compound
A technology of naphthalenesulfonic acid and compounds, which is applied in the field of continuous nitration of naphthalenesulfonic acid compounds, can solve the problems of not knowing the practical application value, not mentioning the reaction effect, heat exchange capacity limitation, etc., to reduce thermal risk and simple structure , the effect of ensuring safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] The nitrated substance A is 1,3,6-naphthalenetrisulfonic acid sulfuric acid solution with a mass fraction of 45%, and the nitrating agent B is a mixed acid containing 80% fuming nitric acid, specifically, 97% fuming nitric acid and 98% fuming nitric acid in the mixed acid % sulfuric acid was prepared according to the mass ratio of 4.7:1, and the temperature of the constant temperature water bath in device C was 50°C. Preheat the nitrated substance A to 50°C, pass the quantitative pump with the nitrating agent B, and enter the circular channel synchronously according to the flow ratio A:B=8.0:1 to react to generate 8-nitro-1,3,6-naphthalenetrisulfonic acid , the nitric acid ratio is 1.2, the residence time is 100s, and the reacted material flows continuously into the receiving bottle. Through HPLC analysis, the conversion rate of the reaction reached 100%, and the selectivity was 99.2%.
Embodiment 2
[0035] The nitrated substance A is 1,3,6-naphthalenetrisulfonic acid sulfuric acid solution with a mass fraction of 55%, the nitrating agent B is fuming nitric acid with a nitric acid mass fraction of 97%, and the temperature of the constant temperature water bath in the device C is 70°C. Preheat the nitrated substance A to 70°C, pass the quantitative pump with the nitrating agent B, and enter the circular channel synchronously according to the flow ratio A:B=6.1:1 to react to generate 8-nitro-1,3,6-naphthalenetrisulfonic acid , the nitric acid ratio is 1.4, the residence time is 60s, and the reacted material flows continuously into the receiving bottle. Through HPLC analysis, the conversion rate of the reaction reached 100%, and the selectivity was 96.8%.
Embodiment 3
[0037] The nitrated substance A is 2,6-naphthalene disulfonic acid sulfuric acid solution with a mass fraction of 35%, and the nitrating agent B is a mixed acid containing 50% fuming nitric acid, specifically 97% fuming nitric acid and 98% fuming nitric acid in the mixed acid Sulfuric acid is prepared according to the mass ratio of 1:0.94. The temperature of the constant temperature water bath in device C is 40°C. The nitrated compound A is preheated to 40°C, and the nitrated agent B enters synchronously according to the flow ratio of 5.7:1 through the quantitative pump. 1-Nitro-3,7-naphthalene disulfonic acid is generated by reaction in the circular channel, the nitric acid ratio is 1.1 and the residence time is 80s. After the reaction, the material flows continuously into the receiving bottle. Through HPLC analysis, the reaction conversion rate reached 99.5%, and the selectivity was 93.7%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com