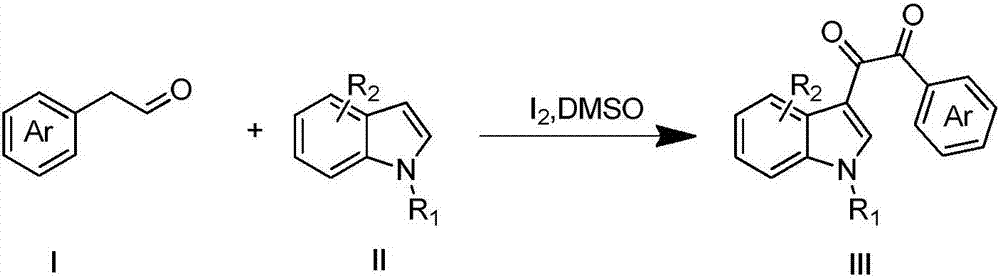
Method for continuously preparing dicarbonyl indole compound by utilizing micro-channel reaction device
A microchannel reaction, biscarbonyl indole technology, applied in chemical instruments and methods, chemical/physical processes, chemical/physical/physical chemical processes, etc. Potential for industrial scale-up, easy operation and control of the preparation process, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
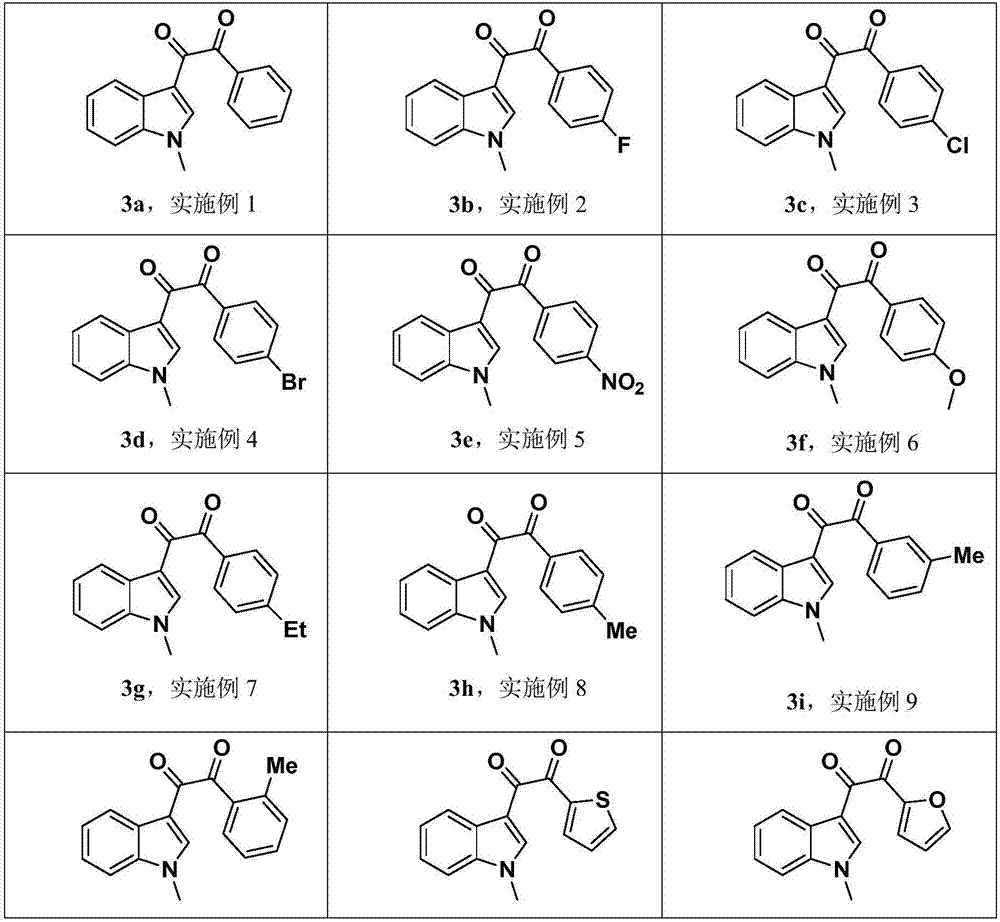
Embodiment 1
[0034] The synthesis of embodiment 1 compound 3a:
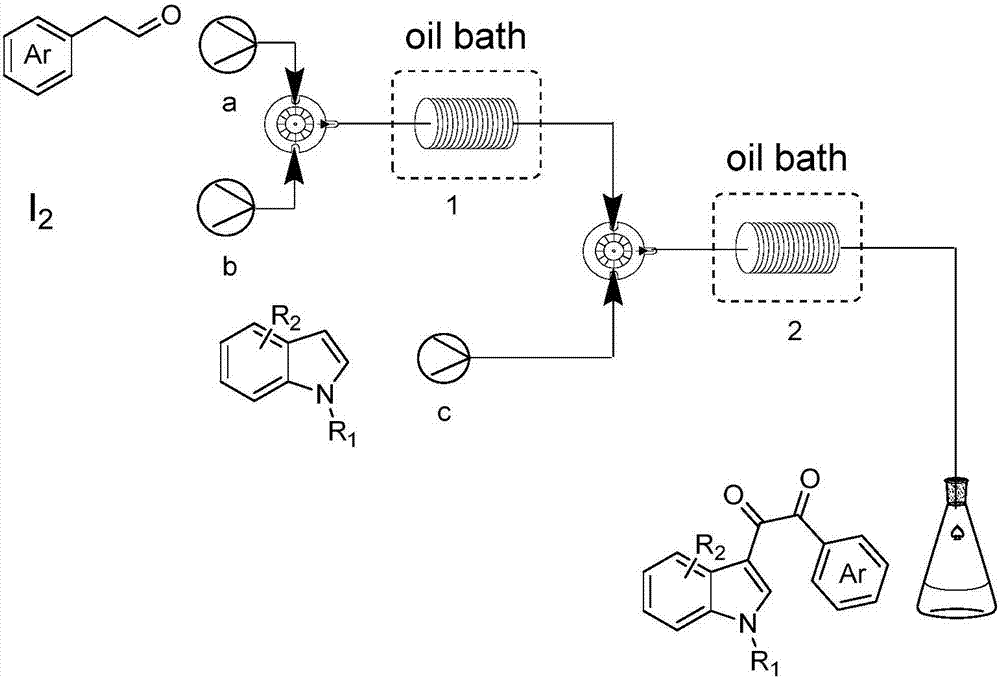
[0035] Dissolve 10mmol (1.20g) phenylacetaldehyde in 10mL dimethyl sulfoxide (dimethyl sulfoxide) to obtain a homogeneous solution A, add it to syringe pump a; dissolve 5mmol (1.27g) iodine in 10mL dimethicone In methyl sulfoxide (dimethyl sulfoxide), a homogeneous solution B was obtained, which was added to syringe pump b; 10 mmol (1.31 g) of N-methylindole was dissolved in 20 mL of dimethyl sulfoxide (dimethyl In sulfoxide), obtain homogeneous solution C, add in the syringe pump c; The injection flow rate of syringe pump a, b is 0.4ml / min, and the injection flow rate of syringe pump c is 0.6ml / min; The first microchannel reaction Reactor reaction volume V=5ml, reaction time 6.25min, the second microchannel reactor reaction volume V=5ml, reaction time 3.57min; The first, second microchannel reactor internal diameter=0.5mm; The first microchannel reactor temperature The temperature of the second microchannel reactor was 110°...
Embodiment 2
[0036] The synthesis of embodiment 2 compound 3b:
[0037] The method is the same as in Example 1, except that the compound I is p-fluorophenylacetaldehyde, the reaction parameters are shown in Table 2, the yield is 85%, and the product 3b is obtained after separation by column chromatography; 1 H NMR (400MHz, CDCl 3 )δ8.51–8.40(m,1H),8.18–8.11(m,2H),7.83(d,J=5.1Hz,1H),7.39(dd,J=5.4,2.6Hz,3H),7.16(t ,J=8.7Hz,2H),3.84(d,J=10.1Hz,3H); 13 C NMR (100MHz, CDCl 3 )δ192.11, 187.16, 167.90, 165.34, 139.78, 137.88, 133.34, 133.25, 130.12, 130.09, 126.55, 124.44, 123.73, 122.80, 116.25, 116.05, 112.92, 122.80, 116.25, 116.05, 112.92, 110.9 ] + Calcd for C 17 h 12 FNO 2 304.0744found 304.0747.
Embodiment 3
[0038] The synthesis of embodiment 3 compound 3c:
[0039] The method is the same as in Example 1, except that the compound I is p-chlorophenylacetaldehyde, the reaction parameters are shown in Table 2, the yield is 82%, and the product 3c is obtained after separation by column chromatography; 1 H NMR (400MHz, CDCl 3 )δ8.52–8.39(m,1H),8.05(d,J=8.6Hz,2H),7.82(s,1H),7.46(d,J=8.6Hz,2H),7.39(dd,J=6.2 ,3.4Hz,3H),3.83(s,3H); 13 C NMR (100MHz, CDCl 3 )δ192.36,186.81,140.95,139.78,137.84,131.99,131.82,129.20,126.50,124.43,123.72,122.77,112.84,110.16,33.92; HRMS(TOF)m / z[M+Na] + Calcd for C 17 h 12 ClNO 2 320.0449found 320.0443.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com