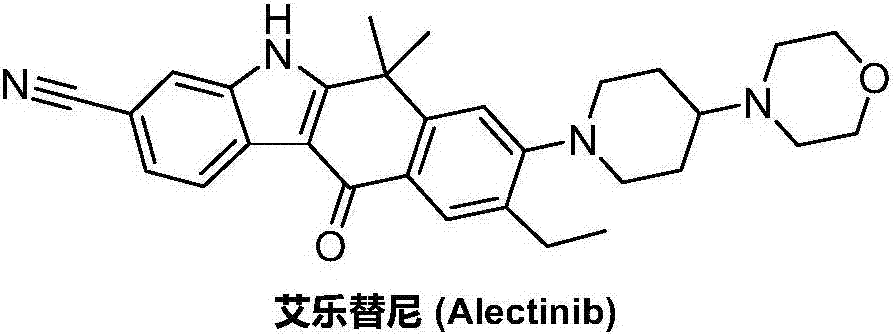
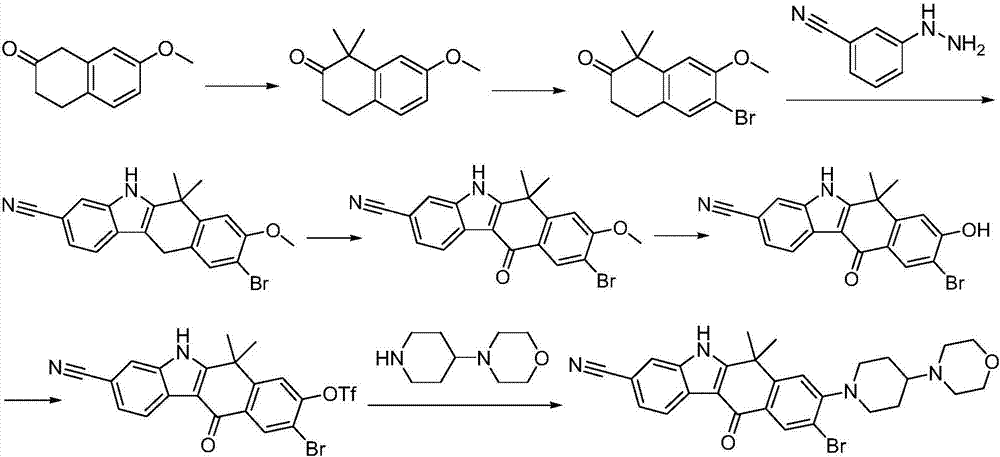
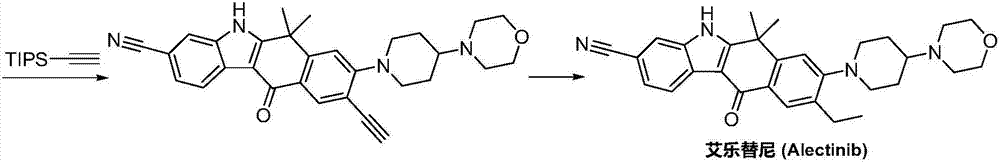
Preparation method of Alectinib
A technology of alectinib and ethyl, which is applied in the field of medicinal chemical synthesis, can solve the problems of short technological process, difficult to obtain, and use a large amount of solvents, and achieves the effects of reasonable technical solution, simplified operation and less impurities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] A) Preparation of tert-butyl 4-{4-ethyl-3-[4-(morpholin-4-yl)piperidin-1-yl]phenyl}-4-methyl-3-oxopentanoate:
[0033] tert-butyl 4-(4-ethyl-3-iodophenyl)-4-methyl-3-oxopentanoate (10.0g, 24mmol) was dissolved in N,N-dimethylformamide (200mL), Add 4-(4-piperidinyl)morpholine (9.2g, 54mmol), sodium methoxide (3.2g, 59mmol), and stir the reaction mixture at 100°C for 12 hours, then cool the reaction solution to room temperature, add water (40mL), and cool Crystallize at -10°C for 3 hours, filter to obtain 4-{4-ethyl-3-[4-(morpholin-4-yl)piperidin-1-yl]phenyl}-4-methyl-3 - tert-butyl oxopentanoate, white solid (9.6 g), yield 87%.
[0034] B) Preparation of 6-cyano-2-{2-[4-ethyl-3-(4-(morpholin-4-yl)piperidin-1-yl)phenyl]prop-2-yl}-1H -Indole-3-carboxylic acid:
[0035] tert-butyl 4-{4-ethyl-3-[4-(morpholin-4-yl)piperidin-1-yl]phenyl}-4-methyl-3-oxopentanoate (9.0g, 20mmol), 3-cyanophenylhydrazine (3.3g, 25mmol) and trifluoroacetic acid (123.1g, 1080mmol) were mixed, th...
Embodiment 2
[0039]A) Preparation of tert-butyl 4-{4-ethyl-3-[4-(morpholin-4-yl)piperidin-1-yl]phenyl}-4-methyl-3-oxopentanoate:
[0040] 4-(4-Ethyl-3-iodophenyl)-4-methyl-3-oxopentanoic acid tert-butyl ester (5.0g, 12mmol) was dissolved in toluene (100mL), and 4-(4-piperidine base) morpholine (5.5g, 32mmol), sodium ethoxide (2.4g, 35mmol), the reaction mixture was stirred at 110°C for 6 hours, the reaction solution was cooled to room temperature, water (50mL) was added, cooled to -10°C for 4 hours of crystallization , filtered to give tert-butyl 4-{4-ethyl-3-[4-(morpholin-4-yl)piperidin-1-yl]phenyl}-4-methyl-3-oxopentanoate , white solid (5.0 g), yield 91%.
[0041] B) Preparation of 6-cyano-2-{2-[4-ethyl-3-(4-(morpholin-4-yl)piperidin-1-yl)phenyl]prop-2-yl}-1H -Indole-3-carboxylic acid:
[0042] tert-butyl 4-{4-ethyl-3-[4-(morpholin-4-yl)piperidin-1-yl]phenyl}-4-methyl-3-oxopentanoate (5.0g, 11mmol), 3-cyanophenylhydrazine (2.0g, 15mmol) and acetic acid (45.8g, 763mmol) were mixed, a...
Embodiment 3
[0046] A) Preparation of tert-butyl 4-{4-ethyl-3-[4-(morpholin-4-yl)piperidin-1-yl]phenyl}-4-methyl-3-oxopentanoate:
[0047] 4-(4-Ethyl-3-iodophenyl)-4-methyl-3-oxopentanoic acid tert-butyl ester (13.5g, 32mmol) was dissolved in 1,4-dioxane (250mL), added 4-(4-piperidinyl)morpholine (10.0g, 59mmol), sodium tert-butoxide (6.2g, 65mmol), the reaction mixture was stirred at 90°C for 18 hours, the reaction solution was cooled to room temperature, and water (120mL) was added, Cool to -5°C for crystallization for 4 hours, filter to obtain 4-{4-ethyl-3-[4-(morpholin-4-yl)piperidin-1-yl]phenyl}-4-methyl- tert-butyl 3-oxopentanoate, white solid (12.6 g), yield 86%.
[0048] B) Preparation of 6-cyano-2-{2-[4-ethyl-3-(4-(morpholin-4-yl)piperidin-1-yl)phenyl]prop-2-yl}-1H -Indole-3-carboxylic acid:
[0049] tert-butyl 4-{4-ethyl-3-[4-(morpholin-4-yl)piperidin-1-yl]phenyl}-4-methyl-3-oxopentanoate (12.5g, 27mmol), 3-cyanophenylhydrazine (4.0g, 30mmol) and formic acid (50.2g, 1091mmol)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com