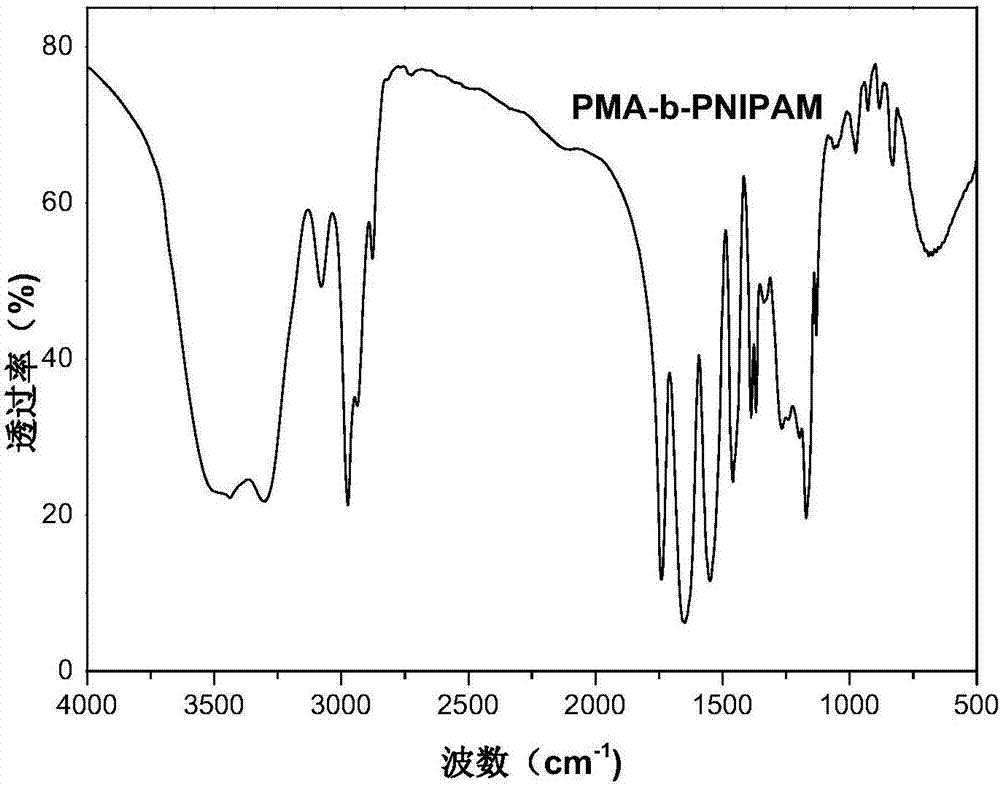
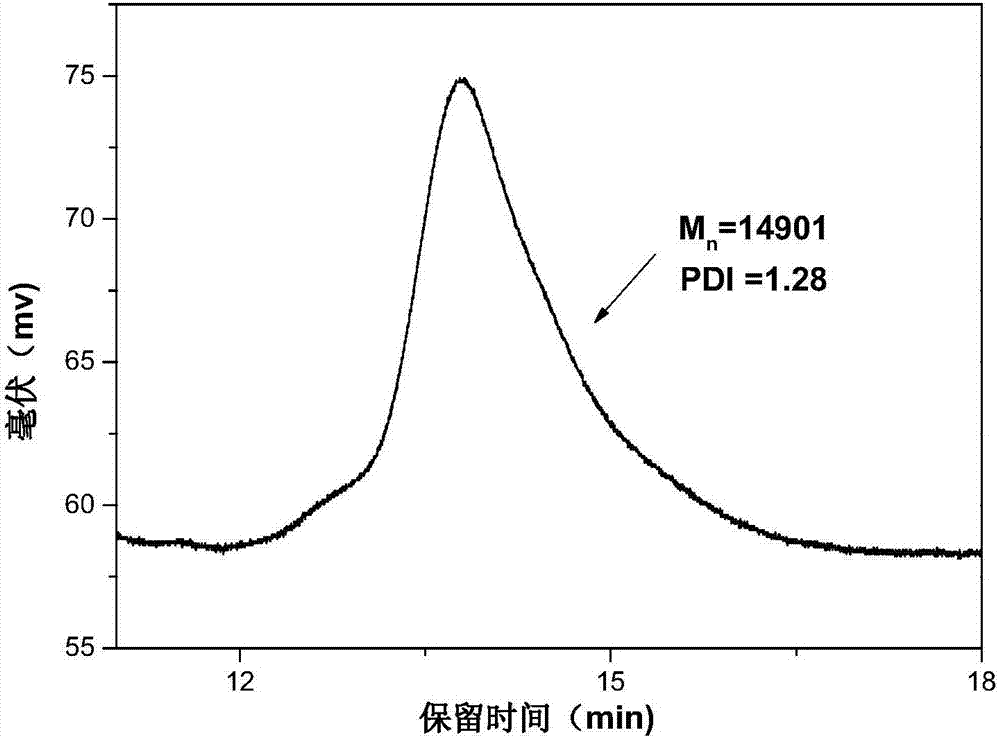
Preparation method for macromolecular adsorbent precursor diblock copolymer
A technology of block copolymer and adsorbent, which is applied in the field of preparation of diblock copolymer as precursor of polymer adsorbent, can solve the problems of widening distribution, uncontrollable reaction, explosion and so on.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] With methyl acrylate (MA) as the first monomer, add 0.05 parts of MA, according to the degree of polymerization n(MA): n(BDMAT)=30, the amount of BDMAT added is 0.0017 parts, azobisisobutyronitrile (AIBN) As an initiator, 1,4-dioxane is used as a solvent, and is added to a round bottom flask, wherein the ratio of the amount of BDMAT to AIBN is controlled at 2:1, and the calculated AIBN added is 0.00085 parts, and the rotor is added to stir Make it completely dissolved, and after passing nitrogen gas for 30 minutes, stir and react in an oil bath at 70°C for 5 hours. After the reaction is completed, quickly put it in an ice bath to cool to terminate the reaction. The yellow liquid obtained after the reaction is precipitated twice in distilled water. To remove the solvent 1,4-dioxane and unreacted monomers, and finally vacuum dry to obtain a yellow viscous liquid polymethylacrylate PMA containing a carboxyl group at one end and a trisulfide bond at the other end 30 -SC(S))...
Embodiment 2
[0028]With methyl acrylate (MA) as the first monomer, add 0.05 parts of MA, according to the degree of polymerization n(MA): n(BDMAT)=30, the amount of BDMAT added is 0.0017 parts, azobisisobutyronitrile (AIBN) As an initiator, 1,4-dioxane is used as a solvent, and it is added to a round bottom flask, wherein the ratio of the amount of BDMAT to AIBN is controlled at 3:1, and the rotor is added to stir to make it all dissolve, and nitrogen gas is introduced for 30 minutes Finally, stir the reaction in an oil bath at 70°C for 5 hours. After the reaction is completed, quickly place it in an ice bath to cool to terminate the reaction. The yellow liquid obtained after the reaction is precipitated twice in distilled water to remove the solvent 1,4-dioxine Hexacyclic and unreacted monomers, and finally vacuum-dried to obtain a yellow viscous liquid polymethylacrylate PMA containing a carboxyl group at one end and a trisulfide bond at the other end 30 -SC(S))S.
[0029] Adopt the met...
Embodiment 3
[0031] With methyl acrylate (MA) as the first monomer, add 0.05 parts of MA, according to the degree of polymerization n(MA): n(BDMAT)=30, the amount of BDMAT added is 0.0017 parts, azobisisobutyronitrile (AIBN) As an initiator, 1,4-dioxane is used as a solvent, and it is added to a round bottom flask, wherein the ratio of the amount of BDMAT to AIBN is controlled at 4:1, and the rotor is added to stir to make it all dissolve, and nitrogen gas is introduced for 30 minutes Finally, stir the reaction in an oil bath at 70°C for 5 hours. After the reaction is completed, quickly place it in an ice bath to cool to terminate the reaction. The yellow liquid obtained after the reaction is precipitated twice in distilled water to remove the solvent 1,4-dioxine Hexacyclic and unreacted monomers, and finally vacuum-dried to obtain a yellow viscous liquid polymethylacrylate PMA containing a carboxyl group at one end and a trisulfide bond at the other end 30 -SC(S))S.
[0032] Adopt the me...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com