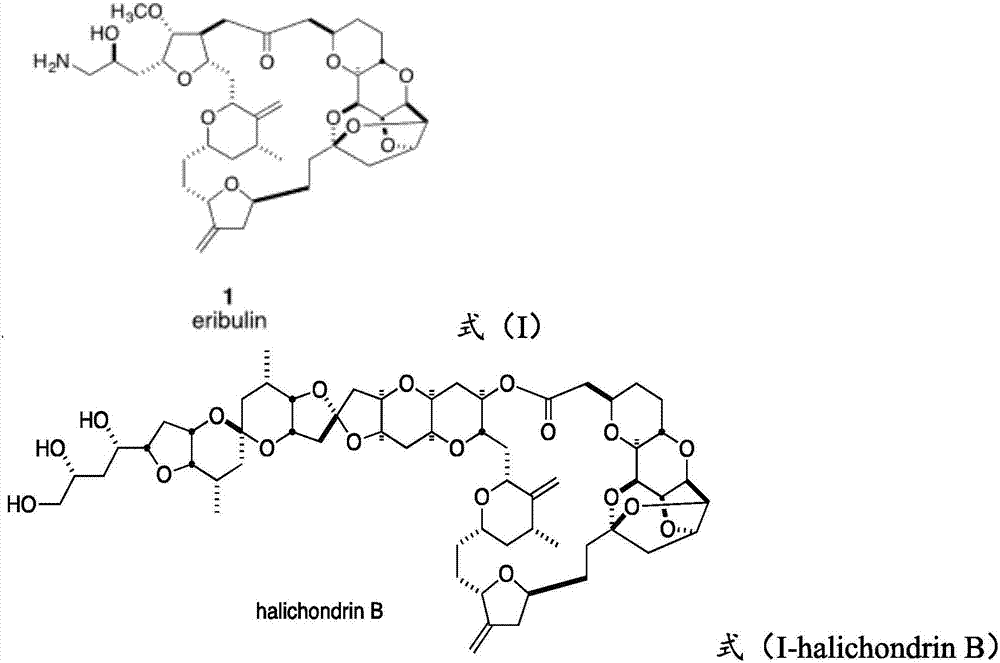
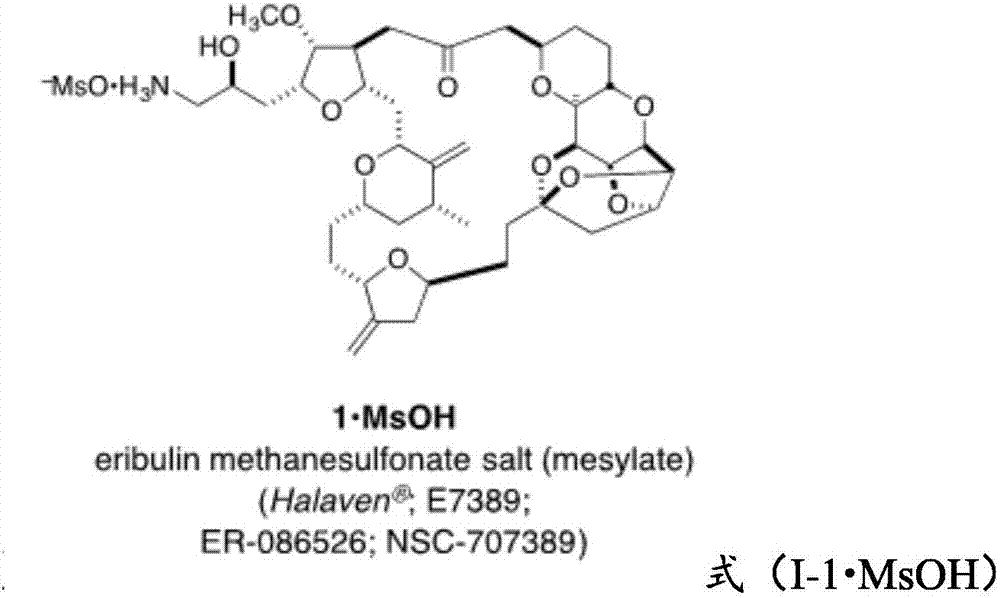
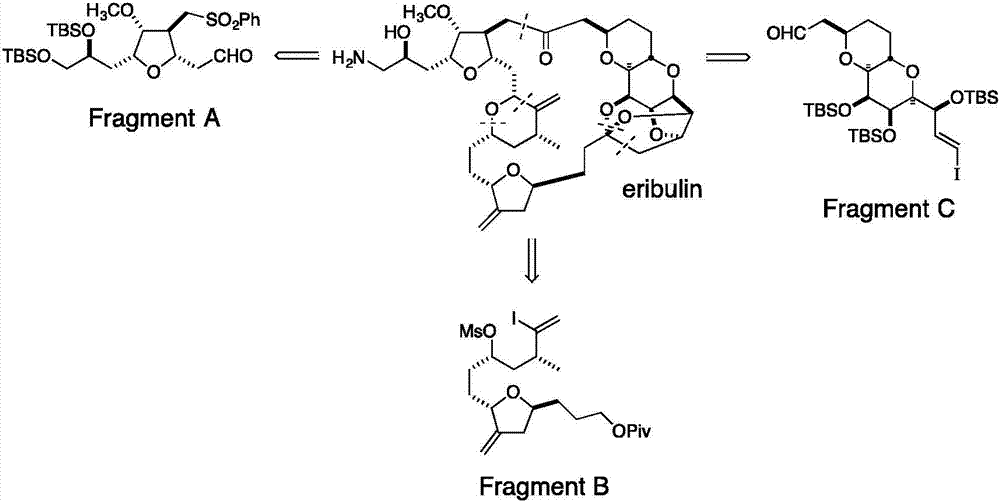
Compound and preparation method and application thereof
A compound and reaction technology, applied in the field of compounds and their preparation, can solve the problems of low reaction yield, influence on eribulin synthesis efficiency, multiple reaction raw materials, etc., and achieve the effect of improving yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0098] The present invention also provides a preparation method of a compound having a structure of formula (II), comprising:
[0099] The compound of formula (III) structure is converted into the compound with formula (II) structure,
[0100]
[0101]
[0102] where the R 1 , R 2 , R 3 , R 4 , R 5 independently selected from C3-C30 alkylsilyl, C7-C30 benzyl or C1-C25 alkyl;
[0103] The Ar is selected from C6-C30 aryl groups.
[0104] According to the present invention, the present invention converts the compound with formula (III) structure into the compound with formula (II) structure, wherein, R in the compound of formula (III) structure 1 , R 2 , R 3 , R 4 , R 5 And Ar is identical with the definition of substituent in intermediate; Among the present invention, the catalyst of described transformation reaction is CrCl 2 / NiCl 2 、Cp 2 Cr / NiCl 2 、CpCrCl / NiCl 2 , Cr(II) / Mg(0) / TMS-X / NiCl 2 , Cr(II) / Zn(0) / TMS-X / NiCl 2 , Cr(III) / Mg(0) / TMS-X / NiCl 2 , Cr(I...
Embodiment 1
[0124]
[0125] Under nitrogen protection, chiral ligand (2.96g, 10mmol) and anhydrous CrCl2 (1.23g, 10mmol) were added to a 50ml three-neck round bottom flask, and 15ml of anhydrous acetonitrile was added thereto with a syringe. The resulting suspension was heated and TEA (1.01 g, 10 mmol) was added dropwise with stirring to give a dark green mixture. At room temperature, the mixed solution was continued to be stirred for 40 min, and NiCl2·DMP (338 mg, 1 mmol) was added thereto, and the color of the reaction solution became dark rapidly. Stirring was continued for 20 min, and the compound of formula (III) (1.588 g, 1 mmol) was dissolved in a mixed solution of 4 ml of anhydrous THF and 2 ml of acetonitrile, and was added dropwise into the reaction solution within 10 min using a syringe. After the dropwise addition, the mixed reaction solution was stirred at room temperature for 1 h, 20 ml of n-heptane was added, and then filtered, and the filter cake was washed with n-hepta...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com