Tumor immunotherapy drug target and application thereof
An immunotherapeutic drug and drug action technology, applied in biochemical equipment and methods, microbial measurement/testing, instruments, etc., can solve the problems of undisclosed associations, etc., and achieve the goal of enhancing antigen-specific immune response and enhancing killing ability Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
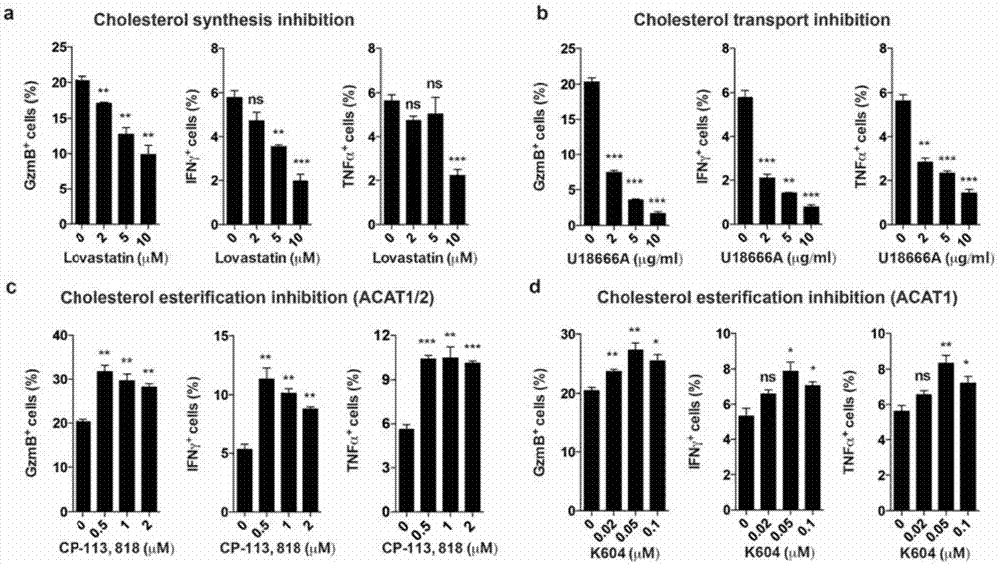
[0230] Example 1 Inhibition of cholesterol esterification can enhance the effector function of CD8 T cells
[0231] Test method: The cells obtained by magnetic bead sorting were resuspended in RPMI 1640 complete medium, and the corresponding concentration of inhibitors was added, and the cells were incubated at 37 degrees CO 2 Cultured in the incubator for 6 hours, then washed 3 times with RPMI 1640 complete medium, and finally resuspended in RPMI 1640 complete medium and transferred to the well plate with activating antibodies α-CD3 and α-CD28, 37 degrees CO 2 The culture was stimulated for 20 hours in the incubator, and then Brefeldin A (5 μg / ml) was added to continue the culture for 4 hours to inhibit the secretion of cytokines. After the culture was over, the cells were collected, labeled with CD8 antibody surface, and then fixed with 4% paraformaldehyde at room temperature for 10 minutes. The cell membrane was punched with 0.1% Triton-X100, and the corresponding proteins ...
Embodiment 2
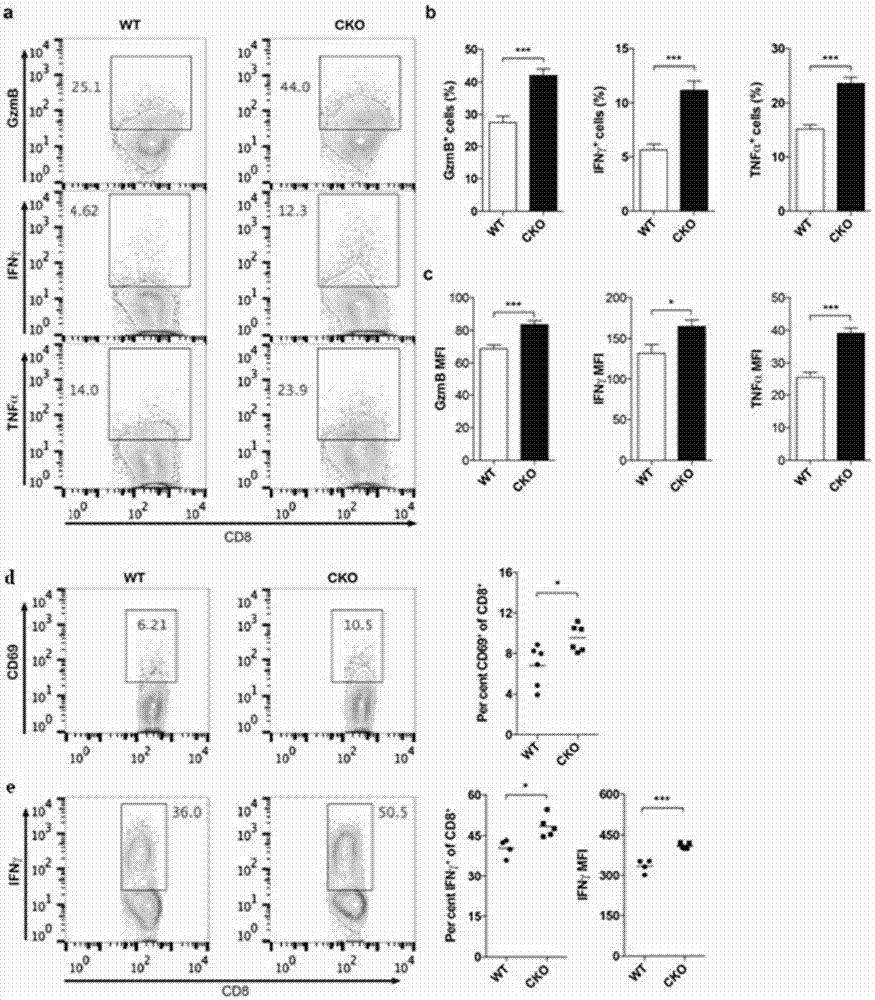
[0233] Example 2 T cell-specific knockout of ACAT1 enhances CD8 T cell immune response.
[0234] The purpose of this example is to verify the function of ACAT1 in CD8 T cells. ACAT1 flox / flox mouse with CD4 Cre The mice were mated to obtain T cell-specific knockout ACAT1 mice (CD4 Cre -Acat1 flox / flox , referred to as Acat1 CKO ) and corresponding wild-type control mice (Acat1 flox / flox ).
[0235] Ex vivo test method: CD8 T cells obtained by magnetic bead sorting were resuspended in RPMI 1640 complete medium, and corresponding concentrations of inhibitors were added, and 37°C CO 2 Cultured in the incubator for 6 hours, then washed 3 times with RPMI 1640 complete medium, and finally resuspended in RPMI 1640 complete medium and transferred to the well plate with activating antibodies α-CD3 and α-CD28, 37 degrees CO 2 The culture was stimulated for 20 hours in the incubator, and then Brefeldin A (5 μg / ml) was added to continue the culture for 4 hours to inhibit the secret...
Embodiment 3
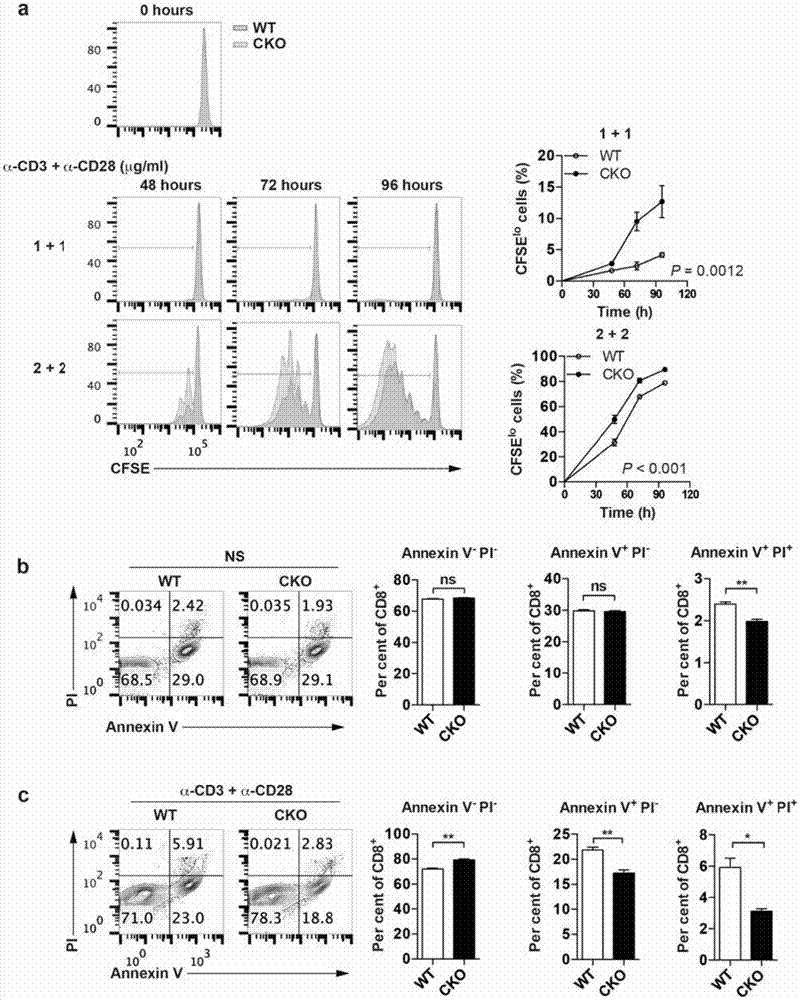
[0238] Example 3 ACAT1 gene knockout promotes the proliferation of CD8 T cells and reduces apoptosis
[0239] The purpose of this example is to detect the effect of ACAT1 on the proliferation of CD8 T cells. Through CFSE labeling experiments, CD8 cells were stimulated with α-CD3+α-CD28 antibodies, and the effect of ACAT1 on the proliferation of CD8 T cells was further detected. Compared with wild-type CD8 T cells, Acat1 CKO The proliferative ability of CD8 T cells was enhanced ( image 3 .a). At the same time, the apoptosis of CD8 T cells after activation was detected by AnnexinV and PI staining, and the results showed that Acat1 CKO CD8 T cell Annexin V - P.I. - Compared with wild-type cells, the proportion of viable cells increased significantly, while cells in apoptotic state (AnnexinV + P.I. - and AnnexinV + P.I. + ) was significantly reduced ( image 3 .b,c).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com